Over the past century, industrial chemists and rubber technologists watched the demand for protective, durable materials climb. Their curiosity led them to work with zinc organic complexes. By the mid-twentieth century, Zinc Di(Benzothiazol-2-Yl) Disulphide—often called ZMBT—started to show up in the toolkit of rubber compounding. My early work in materials science took place in labs still using peroxide and sulfur alone for curing, but as automotive and industrial applications became more demanding, ZMBT began to replace less efficient accelerators. Real advances arrived when researchers learned to control particle size and purity better, making products more predictable batch after batch. Trademark names like Altax, Vulkacit ZnMBT, and Crystex ZMBT started appearing on invoices and spec sheets, and by the 1980s, nobody doubted that this compound had staying power.
ZMBT looks like a pale yellow or off-white powder, free-flowing and light in weight, almost slippery if rubbed between the fingers with gloves. Manufacturers usually sell it in multi-kilo bags labeled with hazard symbols and handling instructions, always focusing on its value as a secondary accelerator in rubber vulcanization. ZMBT fills a very clear niche in keeping the cure temperature low and the cross-linking steady, especially in latex goods and technical rubber products. Its appeal ties directly to the need for latex gloves, condoms, medical devices, and occasionally sealing gaskets that need radiation resistance.
People working with ZMBT quickly notice its mild odor of benzothiazole, though far less pungent than mercaptobenzothiazole itself. Its melting point lies around 170°C or a bit higher, and it remains nearly insoluble in cold water and most solvents, though toluene and chloroform will pull it into solution when warmed. The dust can float and settle everywhere unless proper ventilation exists. Density clocks in near 1.7 g/cm³, and the compound doesn’t burn until exposed to considerable heat, which has always caused safety personnel to enforce strong controls on storage conditions.
Reputable suppliers detail purity, typically above 96% for technical grade. ZMBT usually comes with information on moisture content (targeting below 0.5%), ash content, residue on 63-micron sieves, and assay values. I remember our incoming raw materials logs including both batch number and specific lot certificate. Labels always warn of skin and eye irritation, respiratory sensitivity, and the expected shelf life, which, if kept cool and dry, can extend for two years or more. Abbreviations change depending on region—a supplier in Europe uses ZMBT, while North America often lists CAS and EINECS registration numbers more prominently.
Commerical synthesis of ZMBT usually involves reacting mercaptobenzothiazole with zinc salts such as zinc chloride or oxide in aqueous or mixed solvent systems. Many companies retain older “wet-process” methods but have improved waste stream management to meet today’s stricter environmental monitoring standards. In-house chemistry teams tweak pH and agitation to maximize yield and minimize by-products. I’ve seen R&D chemists push both yields and particle fineness by shifting from batch to semi-continuous processes, though production managers always watch utility bills and effluent treatment requirements when considering upgrades.
ZMBT serves as an intermediate that can participate in further modification, especially for custom accelerator blends. It reacts readily with sulfur donors and can be transformed via standard coupling reactions with other rubber chemicals. In the rubber matrix, ZMBT forms crosslinks by activating sulfur, resulting in improved elasticity and aging properties. Attempts to substitute sodium for zinc during synthesis have only modest benefits for allergy-sensitive users; nothing beats the stability provided by the zinc atom. Processing changes—finer grind, surface treatment to enhance dispersibility—sometimes get marketed as new sub-grades, each with promised improvements tailored to latex or solid rubber.
Chemists, purchasing agents, and production planners know ZMBT under a long list of names: Zinc 2-mercaptobenzothiazole, Zinc salt of MBT, Vulkacit ZMBT, Altax ZMBT, Akrochem ZMBT, and combinations where the “MBTS” confusion sometimes crops up—MBTS being a related but distinct accelerator. All these synonyms trace back through the Benzothiazole family tree, yet most professionals agree on the practical differences these nitrate numbers and subtle formulation changes can make in large-scale production.
Anyone handling ZMBT gets detailed safety instructions—dust inhalation poses a chronic risk, so local exhaust and respiratory protection are standard. Dust devils floating across a compounding room show why these controls matter. Regulatory agencies like OSHA and REACH make regular visits, reviewing worker exposure logs and calling for clean-up procedures if powder accumulates anywhere it shouldn’t. Proper labeling on containers, PPE compliance, and emergency shower/eyewash proximity become a fact of life. One overlooked problem—wearing rings or watches can trap dust and lead to skin irritation if proper washing doesn’t happen post-shift. Many companies now train every technician to recognize ZMBT’s risks from day one.
Demand for ZMBT keeps pace with growth across medical, automotive, and food packaging sectors. Its key edge comes from supporting low-temperature curing of latex and solid rubber, reducing scorch risk, and enabling longer working times. Latex glove production relies on it for meeting performance checks without raising protein degradation. Medical device production depends on accelerator blends where ZMBT occupies the middle ground—neither too slow nor so aggressive as to damage delicate articles. I have seen its impact firsthand on condom lines, where off-cures vanish and rejection rates drop after careful adjustment of ZMBT dosing. Industrial belts, hoses, and gasket producers—always balancing durability against regulatory compliance—keep large stocks on hand for custom compound runs.
Large chemical firms and smaller innovation-driven outfits continue to invest in improving ZMBT properties. Their work targets reducing zinc release, as environmental regulators scrutinize heavy metal leaching from discarded products. Researchers explore co-accelerator systems pairing ZMBT with non-nitrosamine alternatives, hoping to produce safer gloves and food-contact goods. Labs test nano-dispersion to achieve better weather resistance and higher transparency for specialized rubber articles. Graduate students focus on the molecular mechanisms behind ZMBT’s interaction with sulfur and polymer chains, publishing structure-activity correlations that help industry tune formulations while lowering additive loads.
ZMBT, while less toxic than many legacy accelerators, still requires vigilance. Animal studies show some skin sensitization risk, though less severe compared to thiurams or dithiocarbamates. Chronic inhalation studies push for lower workplace exposure limits each decade. Wastewater from rubber plants brings ZMBT’s aquatic toxicity into focus; I’ve seen factory water discharge testing turn up ZMBT breakdown products that regulators now flag for monitoring. Polymer scientists and toxicologists run annual reviews on published toxicokinetics, advocating improvements like contained mixing systems and powder replacement with non-dusting granules.
Emerging trends put pressure on both cost and sustainability, pushing ZMBT suppliers to innovate. Rubber users in the European Union and North America demand cleaner, allergen-reduced, and eco-friendlier products. Potential replacements—magnesium and calcium-based accelerators—haven’t yet matched ZMBT’s balance of cost, performance, and safety. Future technical refinements could see particle engineering and novel surface coatings reduce dust, increase activity, and lower zinc migration. The indicator for real progress remains clear: maintaining ZMBT’s contribution to safety and performance in medical and food-contact applications, even as environmental criteria grow stricter and users look for alternatives carrying a smaller ecological footprint.
Zinc di(benzothiazol-2-yl) disulphide pops up in many technical discussions about rubber production. Chemists and engineers often call it ZMB2S. If you’ve ever held a new car tire or unwrapped a rubber-soled shoe, you’ve run into products shaped by this chemical. ZMB2S belongs to a class of accelerators that influence how quickly and effectively rubber hardens in the vulcanization process. Vulcanization turns sticky, soft rubber into a tough but flexible material that stands up to wear and weather.
The vulcanization process has powered the tire industry, conveyor belts, footwear, hosepipes, and many common goods. Back in the day, rubber’s biggest drawback was its tendency to crack, soften in heat, or become brittle in the cold. By tossing in sulfur and applying heat, Charles Goodyear kicked off a revolution, but controlling that reaction required faster, safer, and more reliable catalysts. ZMB2S steps in as an important accelerator that helps speed up and fine-tune the crosslinking between rubber molecules. Its design increases the efficiency while lowering the energy demand, which lines up with modern industry goals around sustainability and cost savings.
ZMB2S delivers a balance that researchers and manufacturers appreciate. This compound ensures that rubber doesn’t just harden quickly, but develops the right strength, bounce, and resistance to aging. The world’s biggest tire makers have leaned into zinc-containing accelerators to keep up with demand and keep costs in check. Left to slow chemistry alone, each tire would need extra hours in ovens, more energy, and would end up using more raw materials. In a world where every resource and dollar counts, that makes ZMB2S worth talking about.
For all its technical benefits, ZMB2S shares some challenges with other rubber chemicals. The benzothiazole structure, for example, can introduce stability and toxicity issues if not handled with care. Workers in factories face the greatest risk, as repeated exposure or poor ventilation could lead to health impacts. Responsible companies invest in controls—like protective gear, regular air checks, and improved ventilation—to keep people safe on the plant floor. High-profile recalls and tighter government regulation have pushed the industry to reassess both ingredient safety and environmental run-off from production sites.
End-of-life management for rubber remains a hot topic. Tires, in particular, fill up lots of landfills. Few chemicals from the rubber itself leach easily, yet the push continues for safer, smarter additives that break down more easily or offer lower toxicity. Some researchers look at alternative catalysts, but any large-scale shift demands time and new investments. For now, ZMB2S stays in the formula for many rubber blends because it works and there’s a clear safety record when handled correctly.
What lesson lands with ZMB2S applies to most specialty chemicals. Nothing arrives in the toolbox without debate over safety, cost, and function. ZMB2S helps build better tires and products that take daily punishment. Its story proves that chemicals behind the scenes deserve attention—not only for their technical brilliance but for the broader impact on people and the planet. Real progress comes from asking tough questions and building better habits in the lab and on the factory floor.
Zinc Di(Benzothiazol-2-Yl) Disulphide plays a quiet but essential role in the rubber industry, serving as a vulcanizing agent for tires, hoses, and footwear. Many of us rarely stop to consider the journey such a chemical takes before it shows up in our daily lives, disguised as something simple like a shoe sole. Working around specialty additives myself, I’ve seen how careful attention changes everything, especially once hazardous dust or forgotten leaks come into play.
Talk to anyone who’s slogged through warehouses, and they’ll tell you: heat, light, and moisture never make good partners with complex chemicals. Most manufacturers ship Zinc Di(Benzothiazol-2-Yl) Disulphide in thick, sealed drums or multilayer bags. These barriers slow down moisture and air, holding the powder in stable condition. A dry, cool spot, away from direct sunlight, preserves both quality and shelf life. Poor storage ruins investments—the breakdown releases unpleasant odors and even shifts product performance in rubber compounds. Mold, clumping, and foul surprises follow in damp or humid corners. I still remember a supplier call after a leaking roof soaked several pallets; half their raw material arrived clumpy and useless.
Fire safety deserves particular attention. Although this compound doesn’t usually catch fire on its own, it can fuel a blaze if something else ignites. Stack pallets away from oxidizers and open flames. Designate a chemical zone, post hazard signs, and keep spill materials and extinguishers close. Forklift drivers juggling crowded aisles overlook details like loose lids or torn bags, so short walk-around inspections catch potential leaks and contamination long before they ripple out.
Breathing fine powder each day carries health risks most of us only appreciate in hindsight. Skin contact sometimes leads to rashes or allergic reactions, so gloves, goggles, and fitted masks matter more than most realize. In one busy lab setting, I watched coworkers brush dust from their sleeves, only to experience headaches or redness later on. Eye protection gets ignored on quick jobs, though even a small amount in the eyes burns badly and slows down work.
Pouring, scooping, or weighing this material inside a fume hood cuts down airborne dust. Proper ventilation isn’t just a checklist item—it’s the difference between comfort and a persistent cough at the end of a shift. Emphasize clean-up routines; even stray residue along scales and benchtops finds its way onto hands and clothing, hitchhiking home in pockets or on shoes. Soap and water remove visible trace, but deeper cleaning ensures nothing lingers where snacks or coffee cups might go.
Few things expose gaps in safety faster than a hurried job or lack of training. New hires or temp workers often miss out on detailed explanations, so regular walkthroughs and refresher sessions cement habits. Each strong policy gets its strength from routine—the daily actions that catch a loose bag tie or keep aisles clean after a minor spill. Digital tracking systems might automate reminders and inventory checks, but there’s no substitute for eyes-on supervision and real-world know-how.
Regulations from bodies like OSHA and local authorities don’t exist as paperwork burdens; their guidelines grow from hard lessons. Print and post Safety Data Sheets nearby. Make emergency contacts and first-aid gear easy to grab. Encourage crew members to report anything that looks suspicious—early fixes save health and raw material.
Respect for these substances, built from small actions over time, offers returns—cleaner air, less waste, fewer injuries. It’s the sort of value only visible in the details, far from the lab bench or shipping dock, once you step back and look at a safer, more reliable workspace.
Zinc Di(Benzothiazol-2-Yl) Disulphide often turns up in rubber manufacturing, especially in making tires. I’ve seen it referenced in materials lists and safety data sheets at plants where standard wear-and-tear raises questions about long-term risks. Its job centers on acceleration—making rubber vulcanize faster, stronger, and more consistent. Products using this chemical often roll down highways or line factory belts.
Frequent handling of chemicals tends to breed concern around inhalation, skin contact, and cumulative impacts. Direct exposure rarely comes from end products like tires, but workers in mixing or handling operations have more at stake. According to the European Chemicals Agency, repeated or extended inhalation might irritate lungs or eyes. The risk grows if machinery fails or storage leaks—scenarios more common than many companies admit.
Laboratory tests in animals have raised eyebrows; some evidence points to skin sensitization and possible reproductive harm if exposure runs high. That’s where proper ventilation, gloves, and face protection matter. I’ve spent enough time on shop floors to know dust and airborne particles accumulate everywhere you don’t actively vacuum or scrub. Responsible factories educate crews about dangers, but I’ve known crews that cut corners during busy shifts.
Zinc Di(Benzothiazol-2-Yl) Disulphide does not just vanish after use. This chemical can leach from aging rubber or landfilled products, especially as weather and abrasion break down the material. Once outdoors, it breaks apart slowly. Some studies show it harms aquatic life—fish and aquatic invertebrates take the brunt when run-off finds its way into rivers.
On-site spills and mismanaged waste can lead to soil and water contamination. Zinc itself, released in steady doses, becomes toxic to many plants and animals. In my experience speaking with environmental consultants, tire and rubber plants need to prove their discharge stays well below legal limits. Routine water checks and up-to-date treatment systems are the bare minimum.
I believe safer work environments begin with strong housekeeping—aggressive dust collection, regular gear checks, and honest communication between staff and management. Transparent chemical inventories help. Companies should run drills for accidental spills and teach people what to do if something goes wrong.
Stronger barriers between production lines and crew spaces reduce face-to-face time with chemicals. Swapping in green chemistry alternatives shows promise, but replacements must match performance without introducing new challenges. Just swapping one risk for another doesn’t cut it. Collaboration helps—factories, cities, and regulators all bear responsibility.
Zinc Di(Benzothiazol-2-Yl) Disulphide brings real strengths to rubber manufacture but demands respect from users and oversight from regulators. Both work safety and environmental protections have to keep pace with modern production. Regulators update standards for a reason—people and the planet pay when companies slip on safety or discharge. Keeping honest about risks pushes us toward a future with fewer accidents and cleaner waters.
Zinc Dibenzothiazole Disulfide, better known as ZMBT, keeps plenty of rubber manufacturers awake at night. Too much and the batch turns brittle or sticky. Too little and vulcanization drags on or never quite delivers that snap you want from a finished tire, belt, or gasket. Striking the right balance matters for strength, durability, and cost-effectiveness.
In my experience in the industry floor and watching labs experiment for years, ZMBT usually falls between 0.2% and 1.0% by weight of the rubber compound. Sometimes, you see it nudging up toward 1.5% for specialty applications needing extra chemical resistance or heat stability. If you go beyond that, the risk of over-curing climbs fast—something you can tell just by pulling a finished part and flexing it. Nobody enjoys a production recall due to cracking or off-odor from decomposed accelerators.
ZMBT plays a supporting role in most sulfur-cured compounds. On its own, it’s a slow accelerator, but it teams up well with faster friends like MBTS or TMTD to control scorch and flow. I’ve seen batches fail in real time in the press from hasty guesses about dosage. The wrong amount means inconsistent cure rates, which pop up during quality checks: a batch of gaskets that don’t seat properly or a run of footwear soles that split along the seam.
Research supports what old hands in mixing rooms tend to know: the “sweet spot” for ZMBT is a direct line to physical properties. Tensile strength, elongation at break, and reversion resistance all track with precise dosing. Nobody wants to waste raw materials or see rejected lots sent back because someone guessed instead of weighed.
Safety deserves more than a line in the SDS binder. ZMBT dust travels easy, so I’ve worked companies where people ended each shift with sore throats, rashes, or headaches because the loading bay stayed poorly ventilated. Gloves, masks, and proper moving methods help. Even small spills can lead to cross-contamination between batches, so a bit of training and regular cleaning saves time and trouble.
Modern plants don’t rely on just a scale and a scoop. Automated dosing improves accuracy and speed, and I’ve seen output and scrap rates improve the same season new systems roll out. Still, it comes down to keeping staff trained, records tight, and lab tests routine, especially for compounds used in critical applications like medical devices or food-contact products. Regular checks on the balance and calibration catch errors before they cost real money.
R&D teams continue searching for synergists or secondary accelerators that let factories use less ZMBT without giving up performance. Some swap in small amounts of new accelerators with a nod to eco-toxicity and waste disposal concerns. Factories that cut their ZMBT usage without unexpected side effects usually run trial batches first and test for everything from flex life to aroma before making a permanent switch.
Every step from drum to finished part turns on small technical choices, and ZMBT dosage sits near the top of that list. Better accuracy and knowledge save money, protect health, and turn out rubber that performs long after it leaves the plant.
Rubber compounding often feels like solving a complex puzzle. Each chemical brings its own character to the mix, and some just refuse to sit quietly beside others. Zinc Di(Benzothiazol-2-Yl) Disulphide (ZDT), a favored accelerator, often attracts attention in technical circles. It doesn’t just show up as a typical ingredient; it shapes cure rates and impacts the final properties of many rubber products.
Rubber folks lean hard on combinations of accelerators to get the right scorch time, cure rate, and product strength. ZDT steps in as a secondary accelerator, pushing along thiazoles or sulfenamides and even joining hands with guanidines. The mix gets tricky in the lab. Overuse steers compounds toward reversion, where the crosslinks start breaking apart under heat. Vulcanizates might lose strength or flexibility. Some mixers try to stretch ZDT’s influence with DPG (diphenyl guanidine), but the result doesn’t always match the expectation—especially if scorch safety is critical for big, industrial batches.
Rubber compounds almost always contain zinc oxide and stearic acid. This pair supports the vulcanization process, acting as activators. But the balance gets delicate with ZDT in play. Too much zinc oxide can slow cure rates, especially in the presence of ZDT, and lead to unpredictable reversion. Overloading on stearic acid throws off the metal complexing, and in the end, the physical properties start to suffer. Balancing these ratios comes from hands-on testing, not from following textbook tables.
Nobody wants a tire that cracks six months after rolling out of the plant. Antioxidants and antiozonants lend protection, but certain types—like DPPD (di-phenyl-p-phenylenediamine)—can react with accelerators like ZDT. Staining or blooming appears, and that leads to customer complaints. I’ve seen cases where a simple antioxidant switch stopped the blooming issue cold. Sometimes it takes walking the production floor and hearing from quality inspectors to spot these things before they show up in a customer report.
Some rubber goods rely on peroxide cures instead of sulfur-based vulcanization to beat heat aging. ZDT doesn’t go over well in these mixes. It encourages rapid cure with sulfur, but in a peroxide-cured batch, it drags in sulfur contamination and messes with crosslinking. This conflict plays out in poor compression set or surface tackiness, issues that don’t always pop up until field failures pile up. Most shops keep peroxide-cured and sulfur-cured compounds well-separated for just this reason.
A compounder’s job runs deeper than just mixing chemicals. Running tests on the specific compound mix, assessing batch records, and staying in touch with suppliers open the door to catching compatibility issues early. Lab trials catch a lot, but I’ve learned plenty from production teams flagging small shifts in cure time or final appearance. Syncing up with chemical suppliers can uncover adjustments or updated grades that prevent bigger headaches.
People in the industry know no compounding recipe stays permanent. Tweaks and substitutions pop up as raw materials shift or environmental rules get tighter. Open conversations across the lab, production floor, and supplier network make ZDT a helpful ally rather than a source of mystery failures. The more hands and eyes on a formulation, the fewer surprises down the line.
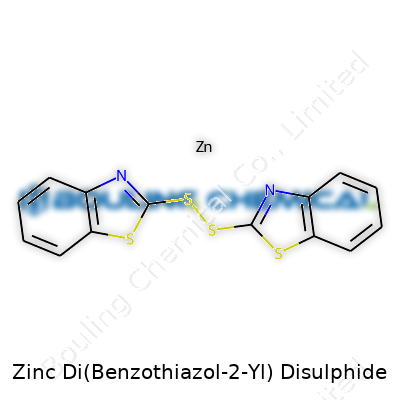
| Names | |
| Preferred IUPAC name | bis(1,3-benzothiazol-2-yl) disulfide |
| Other names |
MBTS MBT Disulfide Benzothiazyl Disulfide Vulcanization accelerator MBTS Bis(benzothiazol-2-yl) disulfide |
| Pronunciation | /zɪŋk daɪ (bɛnˈzoʊθaɪˌzɑːl tuː waɪl) daɪˈsʌlfaɪd/ |
| Identifiers | |
| CAS Number | 155-04-4 |
| Beilstein Reference | 3940896 |
| ChEBI | CHEBI:87756 |
| ChEMBL | CHEMBL3244852 |
| ChemSpider | 2271154 |
| DrugBank | DB11349 |
| ECHA InfoCard | ECHA InfoCard: 100.109.982 |
| EC Number | EC 239-232-0 |
| Gmelin Reference | Gmelin Reference: "Gmelin 116180 |
| KEGG | C18613 |
| MeSH | D015030 |
| PubChem CID | 66256 |
| RTECS number | GF0175000 |
| UNII | O76MDJ1I0N |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C14H8N2S8Zn |
| Molar mass | 574.15 g/mol |
| Appearance | Light yellow powder |
| Odor | Odorless |
| Density | 1.42 g/cm³ |
| Solubility in water | Insoluble in water |
| log P | 3.8 |
| Vapor pressure | 0.0000533 hPa at 25 °C |
| Acidity (pKa) | 2.3 |
| Basicity (pKb) | 6.8 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.68 |
| Viscosity | 3-5 dPa.s (at 60°C) |
| Dipole moment | 0.00 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 573.76 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -146.0 kJ/mol |
| Pharmacology | |
| ATC code | D11AX01 |
| Hazards | |
| Main hazards | H410: Very toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS05, GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | H410: Very toxic to aquatic life with long lasting effects. |
| Precautionary statements | P261, P264, P272, P273, P280, P302+P352, P305+P351+P338, P321, P332+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-1-0-☐ |
| Flash point | > 270°C |
| Lethal dose or concentration | LD50 oral rat: > 8000 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): >5000 mg/kg |
| NIOSH | ENM4011000 |
| PEL (Permissible) | PEL (Permissible): 5 mg/m3 |
| REL (Recommended) | '0.7-1.2 phr' |
| Related compounds | |
| Related compounds |
Zinc dibutyldithiocarbamate Zinc dimethyldithiocarbamate Tetramethylthiuram disulfide Diphenylguanidine N-tert-butyl-2-benzothiazolesulfenamide |