Chemists started searching for better polymer stabilizers way back in the early to mid-twentieth century. Driven by the need to preserve the physical strength and appearance of plastics and rubber, researchers experimented with complex organosulfur compounds. Zinc Di(Benzimidazol-2-Yl) Disulphide started attracting attention as part of this wave of innovation. Labs found that this compound resisted heat and sunlight, slowing down degradation—giving parts made from rubber and plastic a longer working life. Manufacturers latched onto these discoveries, and the material made its way from beaker to production line, showing clear benefit in industrial settings.
Zinc Di(Benzimidazol-2-Yl) Disulphide belongs to a group of zinc-organic chemicals often lumped with antioxidant and UV stabilization agents. Usually appearing as a fine, off-white or slightly yellow powder, it doesn’t smell much and doesn’t flow freely through the hands like table salt—it tends to clump in moisture. Suppliers offer it in drums or big bags, targeting factories that craft hoses, gaskets, seals, or cable insulation. People in research labs spot it under codes and synonyms, such as ZBMD or Benzimidazole Disulphide Zinc Salt.
This material features a molecular formula of C28H18N6S4Zn and a molar mass above 671 grams per mole. The compound holds up well under heat—melting above 300 degrees Celsius—and won’t break down easily in air. You don’t get much odor, and it resists dissolving in water. That last property means it stays in rubber for long periods. Chemically, the sulfur-zinc connections and benzimidazole rings create a lattice good at blocking breakdown from ozone and free radicals, stopping chain reactions that would normally snap apart long molecules in plastics.
Producers document technical specs right on the packaging and safety sheets. They record particle size, purity levels (usually above 98%), and trace heavy metals, which stay well below thresholds set by global health agencies. Factories ordering this stabilizer look for consistency from lot to lot. Each shipment carries batch numbers for tracking and certification for use in industrial processing. Labels highlight hazard codes, disposal instructions, and advice for proper handling, reflecting international standards.
The process usually starts in the lab with benzimidazole, zinc salts, and a sulfur donor—typically thiourea or elemental sulfur. Chemists combine these ingredients under heat in solvents like methanol or ethanol. The reaction forms a precipitate, which workers separate out then wash and dry. Careful drying prevents caking and clumping. Because reactions may release fumes or pose chemical hazards, trained technicians monitor every step, using extraction fans and personal protective equipment to keep the synthesis safe.
Zinc Di(Benzimidazol-2-Yl) Disulphide stands up to a range of tough conditions, but it does interact with strong acids and bases. Introducing oxidizers splits the disulphide bonds, and exposure to certain reactive alloys or solvents can start to break apart the molecule. For companies chasing custom properties in end-products, researchers sometimes tweak the benzimidazole ring or swap the zinc counterion for a different metal, aiming to fine-tune resistance or performance. Each adjustment demands fresh rounds of lab work to make sure toxicity and durability don’t go sideways.
Scientists and purchasing agents run into this compound under names like ZBMD, Zinc bis(2-benzimidazolyl) disulfide, Benzimidazole disulphide-zinc salt, or specific trade names crafted for branded stabilizer mixtures. These often overlap with similar products in catalogs, so researchers double-check specs and safety data.
Prolonged exposure to the raw powder can irritate skin and lungs—standard practice in many plants means gloves, goggles, and air filtration. Safety data sheets advise against eating or drinking while working with the material. Plants train their staff to store it dry and cool with tight lids, cutting down dust and accidental air spread. Regulatory agencies such as OSHA or the European Chemicals Agency dictate limits, so factories stick to process controls and make sure spills get cleaned right up, avoiding drains and soil to protect ground water.
Industrial firms put Zinc Di(Benzimidazol-2-Yl) Disulphide to use in cable insulation, automotive hoses, belts, sealing rings, and tough elastomer blends. It resists the UV rays, ozone, and high heat faced by parts under a car hood, on a factory floor, or outside in the weather. Some labs found that it helps polymer films used in packaging survive months on sunlit grocery shelves. By protecting products from splitting or cracking, it means fewer volume losses to warranty issues and fewer bits of microplastic ending up in the environment.
Labs in universities and private companies still push boundaries with this compound. Research tracks how slight changes in formula can affect stability, strength, or production cost. Focus stays high on alternatives that keep the protective power while shrinking environmental footprint or health risk. Research teams harness techniques like spectroscopy and electron microscopy to map how the stabilizer sits inside rubber blends. As recyclability and life cycle impacts rise on public agendas, R&D teams shift attention to eco-friendly tweaks that won’t lose performance.
Animal studies show that Zinc Di(Benzimidazol-2-Yl) Disulphide possesses low acute toxicity, with little evidence of cancer-causing or chronic harm through skin contact or breathing normal workplace dust levels. Still, researchers keep an eye on long-term exposure and what happens if the compound breaks down in nature. Scientists pore over results for possible hormone effects or bioaccumulation. Regulations track trace impurity levels, and plants test waste streams before sending anything to disposal, aiming to protect workers and the food chain.
Green chemistry is reshaping how companies approach manufacturing and product design. Interest keeps rising for zinc-organic stabilizers that deliver the same punch with less environmental impact. There’s a clear push toward biodegradable or less toxic alternatives, but until those reach the same shelf life and protective properties, Zinc Di(Benzimidazol-2-Yl) Disulphide stays in the mix. In the years ahead, smart chemists and manufacturers will keep hunting ways to blend durability, safety, and sustainability. Strong collaboration between industry and academics stands out as the smartest way to move this material into a future with both performance and responsibility in mind.
Zinc Di(Benzimidazol-2-Yl) Disulphide doesn’t show up in most headlines, yet its real impact shows up in the products folks use every day. This yellowish powder steps out of the shadows in rubber processing plants, where chemists trust it as a key accelerator. Rubber might seem simple at first glance, but behind everything from bike tires to weatherproof seals sits a host of chemicals that shape its look, feel, and performance. Zinc Di(Benzimidazol-2-Yl) Disulphide boosts how rubber cures, making it stronger and more reliable. Without accelerators like this one, a tire could crack just months after hitting the road.
Anyone who’s changed a car tire in sweltering heat knows tough rubber keeps people safe. On the other side, a rubber door gasket failing after a single winter means someone missed a step. Zinc Di(Benzimidazol-2-Yl) Disulphide helps bridge that gap, making sure vulcanization—where rubber transforms from a sticky mess into something sturdy—happens fast and leaves behind a robust material. Checking technical papers and manufacturer reports, I’ve noticed this compound in high-demand where products get put to the test every day: automotive parts, industrial hoses, conveyor belts, even athletic equipment.
This chemical brings certain advantages to the table. Its molecular structure targets sulfur cross-linking, which is essential when crafting rubber that won’t snap or wear out. Some factories pick this accelerator over older chemicals because it tailors itself for low-toxicity demands, which means safer conditions for workers and less environmental worry about what happens when products break down or enter recycling streams. End-of-life tire management continues raising questions, and safer chemicals now play a bigger role in giving answers that regulators and communities can trust.
Anybody managing large-scale production deals with trade-offs. Zinc Di(Benzimidazol-2-Yl) Disulphide costs more than basic accelerators. Smaller firms might hesitate before overhauling a familiar process. Yet, ongoing research from places like the Journal of Polymer Science shows benefits in durability and environmental safety that offset upfront costs over time. Regulatory hurdles sometimes cause headaches. If the European Union tightens chemical rules, factories from Detroit to Seoul might rethink what accelerators they store in their warehouses. This takes investment not just in buying drums of new material but in staff training, quality control, and long-term sustainability audits.
Modern consumers want honest answers about what’s inside the products they touch, chew, or drive. Trust gets built step by step—showing test results, staff certifications, clear recycling programs, and open channels for questions. Applying E-E-A-T means sharing expertise, supporting claims with hard numbers from technical journals and case studies, and admitting where gaps still exist. As more products carry environmental labels or digital QR codes revealing their chemical recipes, factories that clear their books on chemical choices will likely find loyal partners, whether that’s major automakers or local sporting goods stores.
Community voices—workers, engineers, regulators—bring value to discussions on chemical safety and performance. Open forums, workplace surveys, and listening tours can spark new ideas for safer production methods or better end-of-life solutions. Researchers probing the long-term impacts of rubber additives help guide the next generation of manufacturing standards, paving the way for a cleaner, more resilient supply chain. Real-world innovation usually grows from people willing to share what works and why, and Zinc Di(Benzimidazol-2-Yl) Disulphide stands as one more example of chemistry supporting daily life in ways most never stop to notice.
People use Zinc Di(Benzimidazol-2-Yl) Disulphide (ZBMD) in plenty of industrial applications—think of rubber manufacturing, plastics, and even some electronics coatings. In my years handling chemicals in the lab, I’ve seen what happens when shortcuts sneak into the daily routine. This compound is no lightweight: it can irritate the skin, eyes, and respiratory tract. If you value your health, or your team’s, you stay alert around it.
You don’t always get a clear warning before exposure causes problems. ZBMD isn’t a well-known toxin, but it’s smart to treat it with the same respect as anything that could mess with your body. Chemical dust, even if it seems harmless, often leads to long-term discomfort or worse. That’s why I always reach for the right protective gear before a single scoop leaves the jar—even after years of handling compounds like this, complacency has no place.
A datasheet from a trusted supplier outlines the basic risks: skin and eye contact can cause redness or burning. Breathing in particles leads to sore throats, nosebleeds, or a cough that lingers long after work ends. Nobody in the lab wants to end up in the occupational health department with preventable injuries.
There’s no substitute for personal protective equipment when handling ZBMD. Gloves offer a first line of defense—nitrile or butyl styles work best. A regular lab coat won’t stop everything; a long-sleeved, tightly woven one keeps the powder off your skin. Safety goggles block splashes and flying dust—a single eye rub with contaminated hands brings instant regret.
Masking up matters, too. Even if the powder looks heavy and settled, the smallest disturbance lifts particles into the air. A half-face respirator with a P2 or P3 filter keeps out most airborne contaminants. I’ve watched coworkers skip the mask during a quick transfer, only to regret it as they cough through the afternoon.
Good lighting and steady ventilation clear lingering dust before it can build up. Fume hoods do the heavy lifting here, pulling hazardous air away from your face. Between busy shifts, a thorough clean-down cuts the risk for everyone who shares the space. Simple habits, like washing hands before you leave the bench or not bringing snacks into the lab, keep accidental ingestion off the table.
ZBMD deserves a safe, dry home. Workers should store it in airtight, clearly labeled containers far away from incompatible materials such as acids and oxidizers. Separate chemical storage cabinets prevent cross-contamination, which helps avoid surprises nobody wants to explain to the safety officer.
I always keep an emergency eye-wash station within arm’s reach when handling unfamiliar chemicals. If spills or splashes happen, quick access makes the difference between minor irritation and a serious medical visit. Every lab or workshop using ZBMD should drill spill response procedures and make sure everyone knows the escape route. Emergency numbers belong on the wall, not buried in a binder.
Trust me, the best stories aren’t those involving hospital trips—they’re the ones where everyone goes home healthy. Respecting ZBMD and similar compounds doesn’t slow the work down; it puts you in control and builds trust within your team. Staying safe is a choice worth making every day.
Zinc Di(Benzimidazol-2-Yl) Disulphide has crept its way into labs and factories as an accelerator in the rubber industry and as a stabilizer in plastics. Folks in manufacturing know it for boosting performance of end products, but green thumbs and cautious parents see chemicals like this as potential hazards. As environmental awareness grows, industries face sharper questions about used compounds—not just how they work, but what they leave behind.
It helps to picture how this compound shows up in the environment. Runoff from plants, scrap heaps, worn-out tires—these are real channels for zinc and synthetic organics to leak into water, soil, and air. Benzimidazole rings make it less biodegradable and more likely to stick around than more natural alternatives. There’s little public data on exactly how long it lingers, but similar structures in the benzimidazole family resist breakdown and can travel through food chains. That's a red flag for those of us worrying about groundwater safety or the health of frogs and fish downstream.
Zinc isn't unfamiliar. It pops up in multivitamins, crops, and animal feeds. Plants and creatures need it, but too much upsets ecosystems. Zinc from rubber accelerators isn't pure zinc—it brings along organic compounds that can persist. Studies from the U.S. Geological Survey and academic outfits abroad link zinc runoff to stunted plant growth and risk of bioaccumulation. Regulations such as REACH in Europe nudge companies to produce more eco-friendly chemicals, but lagging research on new synthetic compounds leaves gaps. Real-world effects often go unseen until local waterways show rising metal levels or aquatic populations dip.
Rubber producers can turn to safer accelerators like thioureas or use more traditional methods that create less waste. Scientists keep hunting for biodegradable alternatives, sometimes tweaking plant-based molecules to do the same work as bigger synthetic ones. Results vary, but momentum is shifting as supply chains ask for cleaner records and downstream users—car companies, toy makers—prefer less risky additives for their own compliance and branding.
I’ve talked with engineers in polymer plants who know a chemical like this can shave costs but also push up long-term risks. Cleanup crews have little patience for persistent residues. Over time, the pattern repeats: industry spots a shortcut, then spends years dealing with unintended consequences. Those of us who care about clean water see substitutions start with pilot projects, then build as testing catches up.
Safer chemistry happens through honest audits and better testing. Industries that map their discharges and swap stubborn compounds for greener ones get ahead of future regulations—and protect their communities. Open disclosure on environmental behavior gives everyone—from regulators to neighbors—a clearer sense of what enters their yards and streams. Governments and researchers can push responsibility, but those closest to the problem usually spot the best fixes. Refusing to treat any synthetic as harmless, and insisting on proper risk checks, sets the stage for a smarter, cleaner approach.
Zinc Di(Benzimidazol-2-Yl) Disulphide—quite a mouthful, but the name gives away its makeup if you break it down. The core here is zinc, a metal many folks associate with sunscreen or the shiny bits in hardware. The zinc atom in this compound connects to two sulfur atoms, each tying into a benzimidazole group. Bennzimidazole itself, shaped by fusing benzene and imidazole rings, brings some real chemistry muscle, often showing up in biological and industrial settings.
Stack the atoms in your mind, and you’ve got zinc at the center, two “arms” of sulfur, and at the end of each, a benzimidazol-2-yl group. The sulfur atoms act like strong glue between the metal and the benzimidazole, not just linking them, but steering the overall shape. This structure isn’t just for show—shape means everything in chemistry, because it tells us how the molecule behaves in the real world.
Benzimidazole rings aren’t newcomers. They appear all over pharmaceuticals and fungicides, thanks to their stable, reactive nature. Combine them with a metal like zinc, hitch them to sulfur, and something unique happens. This fusion creates a compound that doesn’t just resist breaking down, but also holds up under rough conditions—heat, light, and certain chemicals. These properties catch the eye in industries where stability counts, like rubber manufacturing.
I’ve seen this approach in action. Years ago, a rubber plant looking to cut wear and tear on their conveyor belts began turning to zinc compounds like this one. They found their belts lasted longer and showed fewer microbial problems. Turns out, the structure of Zinc Di(Benzimidazol-2-Yl) Disulphide brought more than durability; it offered support against fungi and other decomposers that cut life-spans of common materials.
Compounds that blend metals, aromatic rings, and sulfur can raise eyebrows for good reason. Zinc plays an essential biological role, but too much entering the environment can harm water supplies and ecosystems. Sulfur and benzimidazole derivatives have their environmental footprints. If factories don’t monitor and control waste, compounds like this can wind up in places they shouldn’t be.
Regulators and producers face the job of keeping use and disposal in check. Best practice leans on closed-loop systems, reclaiming chemicals from process streams, and safe incineration where needed. Researchers push to design “greener” versions, tweaking ring structures or swapping out certain connections to cut long-term impact. Real transparency about ingredient sourcing, manufacturing emissions, and waste handling helps customers make choices they stand behind.
Chemists keep searching for substitutions that deliver on toughness and resistance, but break down without leaving a mark when their jobs are done. “Environmentally intelligent design” has shifted from buzzword to reality as companies realize customers look at more than price and performance. Responsible sourcing for zinc, careful management of sulfur emissions, and clear communication can all form part of a cleaner roadmap.
Looking at compounds like Zinc Di(Benzimidazol-2-Yl) Disulphide, anyone connected to chemistry—whether in research, regulation, or manufacturing—has a stake in how structures and supply chains play out. Not just for end users, but for future generations living downstream.
Zinc Di(Benzimidazol-2-Yl) Disulphide finds its main role in rubber production. Tire and rubber producers count on it to protect their products from premature degradation. This chemical delays aging caused by heat, oxygen, and sunlight. Think about tires rolling across hot pavement for tens of thousands of miles—years of exposure create the perfect storm for cracking and breakdown. Compounds like this help tires keep their flexibility and durability.
Rubber hoses, belts, seals, and gaskets benefit in the same way. Anyone who has replaced a crumbly old belt under the hood or seen a dried-out gasket knows that small components often fail far earlier than the parts they serve. Factories blend this disulphide into the rubber mix to slow that process, meaning less frequent replacements and greater product reliability. It’s especially valued in car and truck tires, conveyor belts in heavy industry, and rubber goods exposed to outdoor conditions.
The auto industry invests a lot in keeping components working in tough environments. From O-rings to vibration dampers and bushings, engineers look for ways to stop oxidation and mechanical wear. Zinc Di(Benzimidazol-2-Yl) Disulphide not only extends the lifespan of these products but also supports the creation of lower-maintenance vehicles.
Automotive suppliers favor chemicals that work well with modern rubber formulations, especially those formulated to meet environmental and performance standards. Testing shows that this material lowers the rate of hardening and cracking, even in extreme cold or high humidity.
Cables need to last decades, underground or bundled inside walls. Without chemical protection, the flexible rubber coverings would quickly dry out and crack apart. This disulphide preserves the insulating properties of cable jacketing by slowing down reactions that lead to breakdown. As a result, manufacturers see fewer failures and avoid costly callbacks.
It’s not just in household wiring—mining operations, telecommunications, railway power lines, and offshore oil rigs all depend on stable, long-lived insulation. In each case, premature cable breakdown risks safety, lost productivity, or even environmental disaster.
Although rubber processors use the bulk of Zinc Di(Benzimidazol-2-Yl) Disulphide, plastic manufacturers rely on it for niche uses. Films, container linings, and some specialty plastic parts get the same antioxidant protection. Some packaging designed for chemicals or long-term storage—a drum lining, for example—uses this additive to lengthen functional life.
A common problem in plastic goods is discoloration and brittleness from sun or heat. By adding a proven antioxidant, companies deliver goods that hold up longer in the field, pleasing both clients and regulators.
Rubber and plastic waste is a growing concern. Prolonging the life of tires, cables, or plastic linings means less discarded material, reducing pressure on landfills and cuts down on raw material demand. Extending product lifespans is one way to reduce waste, lower maintenance costs, and support greener supply chains.
Looking ahead, researchers explore new blends that combine strong aging resistance with less environmental impact. Some experiments use this disulphide alongside next-generation stabilizers to push performance further, responding to both tougher usage and stricter regulation.
From a hands-on perspective, every time a machine keeps running with a decades-old belt, or a car passes an inspection with its original bushings, some credit goes to chemists who thought about the long run.
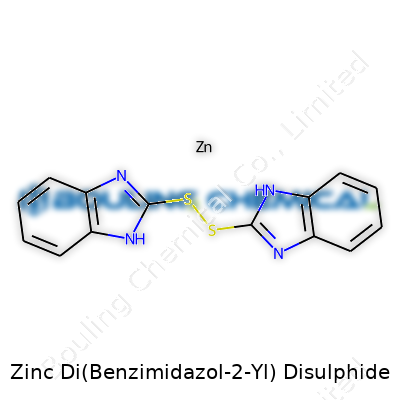
| Names | |
| Preferred IUPAC name | bis(1H-benzimidazol-2-yl) disulfanylidenzinc |
| Other names |
BIS(BENZIMIDAZOL-2-YL) DISULFIDE Zinc salt of BIS(2-BENZIMIDAZOLYL) DISULFIDE ZMB2 BIS(2-BENZIMIDAZOLYL) DISULFIDE ZINC SALT Zinc dibenzimidazolyl disulfide Bis-benzimidazole disulfide zinc salt |
| Pronunciation | /ˈzɪŋk daɪˌbɛn.zɪ.mɪd.əˌzɒl tuː aɪ dɪˈsʌl.faɪd/ |
| Identifiers | |
| CAS Number | 61617-00-3 |
| Beilstein Reference | 2636427 |
| ChEBI | CHEBI:132755 |
| ChEMBL | CHEMBL1996852 |
| ChemSpider | 32239155 |
| DrugBank | DB11451 |
| ECHA InfoCard | 03c99492-ae43-447b-ad29-9ea0b07e53b3 |
| EC Number | 261-236-5 |
| Gmelin Reference | 78778 |
| KEGG | C18606 |
| MeSH | D000397 |
| PubChem CID | 139399358 |
| RTECS number | GF1820000 |
| UNII | I95L9IT9H7 |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID3039240 |
| Properties | |
| Chemical formula | C14H10N4S3Zn |
| Molar mass | 526.72 g/mol |
| Appearance | Light yellow powder |
| Odor | Odorless |
| Density | 1.42 g/cm³ |
| Solubility in water | Insoluble in water |
| log P | 5.28 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 9.45 |
| Basicity (pKb) | 10.85 |
| Magnetic susceptibility (χ) | -0.85 x 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.78 |
| Viscosity | 800-1200 CPS |
| Dipole moment | 4.52 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | Std molar entropy (S⦵298) of Zinc Di(Benzimidazol-2-Yl) Disulphide is 594.44 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | ΔfH⦵298 = -270.6 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | D10AB05 |
| Hazards | |
| Main hazards | May cause damage to organs through prolonged or repeated exposure. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. Harmful to aquatic life with long lasting effects. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H410: Very toxic to aquatic life with long lasting effects. |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > 250°C |
| Lethal dose or concentration | LD50 (Rat, oral): > 8000 mg/kg |
| LD50 (median dose) | Oral LD50 (rat): >2000 mg/kg |
| PEL (Permissible) | 15 mg/m3 |
| REL (Recommended) | 0.5 – 2.0 phr |
| Related compounds | |
| Related compounds |
2-Mercaptobenzimidazole Zinc dibutyldithiocarbamate Zinc dimethyldithiocarbamate Zinc 2-mercaptobenzothiazole Zinc bis(O,O-diethyldithiophosphate) |