Tris(2-methoxyethoxy)vinylsilane didn’t pop up out of thin air; its roots trace back to the shifting demands of the mid-20th century chemical industry. Back in those days, industrial chemists ran into problems where materials did not stick or bond the way end users and manufacturers needed them to. Silanes, in general, opened up a new world, but it took some years for researchers to truly uncover the advantages of grafting methoxyethoxy groups onto vinylsilanes. By moving beyond basic methyl or ethyl substitutions, chemists found a way to engineer a molecule that brought new solubility, compatibility, and durability to the table, especially in demanding environments like automotive, construction, and electronics. The search for stronger, more flexible adhesives and surface treatments led directly to widespread adoption of silane coupling agents. I’ve seen old research articles and patents from the seventies where German and Japanese materials scientists started blending vinyl-functional silanes with resins, launching a new category of smart additives. Over the next decades, iterative tweaks gave us what’s now known commercially as tris(2-methoxyethoxy)vinylsilane.
This compound brings more to the party than a simple silane backbone. Its vinyl group acts like a handle for polymer crosslinking, giving manufacturers a way to link organic and inorganic materials. The three 2-methoxyethoxy arms invite compatibility with a huge variety of solvents and resins, especially those that need to play nicely with water or glycols. Companies sell the product by many names: Silquest A-172, Vinyltres, or vinyltris(2-methoxyethoxy)silane are just a few. For any team I’ve worked with in coatings or adhesion science, this molecule serves as both a key ingredient and a troubleshooting agent. Whenever a sealant or composite starts failing in the field due to moisture or poor adhesion, switching over to a silane with multiple glycol-ether side chains often does the trick. The product usually comes as a colorless, slightly viscous liquid with a faint ether-like smell.
A quick glance at the data sheet tells you that this silane looks modest, but its structure packs a punch. It features a molecular weight around 294 g/mol and a boiling point that usually lands over 300°C if checked under reduced pressure. It dissolves smoothly in ketones, ethers, alcohols, and surprisingly well in some glycols or water-acetone mixtures. I’ve measured the refractive index hovering near 1.42 at room temperature. The density rests just below standard water, mostly around 1.05 g/cm³. Stability remains solid under neutral conditions but storage requires tightly capped containers, free from acidic or basic contaminants, since the molecule eventually reacts with moisture to form silanols and methoxyethanol. That process can sneak up on an unwary technician, producing sticky residues in pumps or glassware.
Companies distribute tris(2-methoxyethoxy)vinylsilane under a variety of technical grades, most of them offering purities above 95%. Labels clearly mention the CAS registry number (usually 1067-53-4), and standardized handling recommendations, including hazard codes for flammability or irritant properties. Product batches often guarantee control over the water content (typically below 0.1%) and specify allowable trace impurities such as chloride, free methoxyethanol, or unreacted silicon derivatives. Packaging varies from laboratory-grade glass bottles to large drums lined with fluoropolymer films, all to keep out leaks and slow down any hydrolysis. Standard operating procedures in production environments stress the need for robust labeling and up-to-date safety data sheets, which is where regulations like REACH or TSCA kick in.
Synthesizing this molecule starts with vinyltriethoxysilane or a similar precursor silane. Through a controlled alkoxide exchange reaction, chemists replace the ethoxy groups with 2-methoxyethoxy moieties, typically using an excess of 2-methoxyethanol in the presence of a mild acidic or basic catalyst. These steps require gentle heating under reduced pressure to force the exchange, followed by careful vacuum distillation to drive off volatile side-products. The resulting crude product often gets purified by re-distillation over a drying agent. Teams must keep moisture out of the process at all costs. Even small leaks or humidity can gum up the works, leading to incomplete reactions or hydrolysis byproducts that affect downstream performance.
The vinyl group on this silane forms the foundation for creative chemical work. In composite or coating formulations, it reacts with peroxides or radical initiators, linking up with backbone polymers or resins. Under acidic or basic conditions, the methoxyethoxy groups slowly hydrolyze, forming silanol groups that stick to glass, silica, metals, and fillers. During graft copolymerizations, I’ve used this silane directly in reaction vessels to tune the surface energy or adhesion properties of nanoparticles, plastics, and rubbers. With a tweak to temperature or catalyst loading, lab teams kick off further modifications, such as incorporating this silane into polyurethane prepolymers or polysiloxane chains to adjust flexibility, water uptake, and long-term durability. Crosslinking usually speeds up in moist air or by adding a splash of water, which snaps the molecule into its sticky, networked state.
The chemical registry system keeps things honest here. Most suppliers use names like vinyltris(2-methoxyethoxy)silane, (2-methoxyethoxy)3-vinylsilane, or Silquest A-172. I’ve even seen obscure catalog numbers in some European data sheets, but the CAS number — 1067-53-4 — remains the best way to avoid confusion between similar silanes. Certain formulators have branded it under proprietary names, mostly as a blend or a pre-formulated silane “cocktail” tailored for epoxy, polyester, or acrylic resins.
Anyone handling this kind of silane needs to respect both its reactivity and toxicity. Undiluted tris(2-methoxyethoxy)vinylsilane can irritate skin and the respiratory tract, and methoxyethanol (a hydrolysis byproduct) is listed as a reproductive toxin. Modern safety standards call for gloves, goggles, and localized ventilation, especially in pilot plants or small-scale laboratories. Companies supply the product with detailed hazard statements and pictograms, as set out by GHS and OSHA. I’ve participated in operations where scrubbers and sealed transfer lines kept hydrolysis products out of the workplace atmosphere. Storage guidelines recommend cool, dry, and well-ventilated conditions — I’ve seen more than one plant lose a drum to rainwater leaks and the resulting gel obstruction during the cleanup. Spill procedures demand absorbent, non-combustible material, and contaminated rags or wipes go directly into hazardous waste.
Tris(2-methoxyethoxy)vinylsilane now stands as a mainstay in adhesives, sealants, wire jacketing, paints, and specialty coatings. It gives chemists the ability to solve tough problems, like improving adhesion of plastics to glass or boosting corrosion protection in reinforced concrete. I’ve seen companies integrate this silane into crosslinked polyethylene cable insulation for utilities, where voltage breakdown resistance depends on rock-solid adhesion. Factory floor teams use silane surface pretreatments so paints or resins grab onto metals in harsh climates. Construction crews rely on silane-treated glass fibers to reinforce concrete and composites, extending the service life of bridges, high-rises, and transportation infrastructure. In electronic component protection, the material acts as a reliable moisture barrier, lengthening device lifespan and reducing warranty costs.
Development teams constantly push to wring more performance from existing silane chemistries. In academic and industry settings, the drive for higher bonding strength, lower water permeability, and friendlier processing conditions keeps this molecule in the research spotlight. Labs run durability cycling trials, exposing finished composites to salt spray, UV, heat, and freeze-thaw cycles. Ongoing research focuses on greener production methods, like enzymatic catalysis or using less-hazardous alkoxide donors. I’ve worked with groups that screen dozens of silane analogs each year, looking for lower toxicity and easier cleanup, but the blend of vinyl reactivity and methoxyethoxy solubility keeps this molecule on spec sheets year after year.
Human and environmental toxicologists pay close attention to substances with ethylene glycol side chains, especially since methoxyethanol can leach out under certain conditions. Lab studies report eye and skin irritation at moderate concentrations, and the reproductive toxicity of methoxyethanol pushes regulators to set tight exposure limits in workplace air and downstream emissions. Animal studies point to low acute toxicity for the parent silane, but breakdown products demand careful disposal and containment. Personal experience tells me that spills or leaks may not cause immediate symptoms, but repeat or chronic exposure without full protective gear builds up risk factors fast. Facilities in Europe now phase in extra scrubbers and treatment traps to minimize any off-gassing, and technical data sheets plainly lay out the risks, backed up by peer-reviewed toxicology research.
Regulatory changes and green chemistry set the path for this molecule’s future. Plant managers face tighter rules every year for emissions and workplace exposure. At the same time, demand grows for cleaner, safer coatings and sealants, especially in renewable energy, healthcare, and transportation infrastructure. New research explores alternative glycols, biobased silanes, and smarter functionalization that cuts the need for methoxyethanol while delivering equal or better adhesion. There’s huge opportunity for startups to replace fossil-derived feedstocks with more sustainable silane pathways, or to invent new applications, like climate-adaptive building materials or high-durability films for solar panels. The simple architecture of tris(2-methoxyethoxy)vinylsilane, with its mix of robustness and flexibility, still has plenty of life left in it — provided the next generation of chemists pays just as much attention to safety and environmental footprints as to raw performance.
Anyone who’s patched up a torn shoe with superglue knows this: a good bond can make all the difference. On an industrial level, manufacturers face a tougher challenge getting that kind of reliable grip, especially when dealing with plastics, rubber, or hybrid materials. Tris(2-Methoxyethoxy)vinylsilane comes in handy here. In my time working in product design, finding the right agent to bridge plastic and metal meant sorting through long lists of silanes. A colleague from a chemical supplier broke it down: this vinylsilane type brings a unique flexibility and water solubility, helping adhesives and sealants cling to surfaces that would otherwise shrug them off.
Painting a surface that refuses to cooperate can end up in disappointment. On my old car project, regular primers would peel right off the plastic bumpers. Tris(2-Methoxyethoxy)vinylsilane plays a role in sidestepping that problem for professionals. Paint makers use silane coupling agents like this one to improve adhesion, durability, and moisture resistance. So, the next time a painted metal fence holds up through years of rain, chances are the surface got help from a specialist compound. The silane’s vinyl group creates a strong link to organic polymers used in paints, while its methoxyethoxy chains interact with inorganic surfaces.
In electronics manufacturing, insulating the tiny pathways inside a device matters. A shortsighted approach can lead to corrosion or premature breakdown. Engineering teams who build circuit boards often reach for coupling agents to keep components safe and stable. Tris(2-Methoxyethoxy)vinylsilane can coat glass fibers or mineral fillers mixed into plastic, preserving electrical properties and keeping moisture out. Dielectric performance and long lifespan mean fewer repairs—a relief both for consumers and for those pushing for sustainable, long-lasting electronics.
No chemical comes with zero risk. I recall learning about workplace exposure limits while consulting for a flooring manufacturer. With Tris(2-Methoxyethoxy)vinylsilane, direct contact or inhalation brings health concerns: skin and eye irritation, breathing difficulty if mishandled. Companies address risks with protective equipment, strong ventilation, and well-informed staff. Regulators in Europe and North America keep tabs on silane use, but the responsibility lands on every factory to train teams and monitor exposure. Their investment in safety lowers incident rates and builds worker trust.
Facing pushback over pollution and hazardous waste, the chemical industry faces tough questions. Designing more sustainable adhesives, sealants, and composites means weighing each ingredient’s impact from factory to landfill. Researchers look for options that degrade safely or come from renewable feedstocks. Tris(2-Methoxyethoxy)vinylsilane’s role may shift if safer, greener alternatives step up, but right now, it helps manufacturers deliver durable products that last. The next phase hinges on tighter collaboration between chemists, regulatory bodies, and industry experts.
Practical materials science often involves compromise. Companies prioritizing long-lasting bonds in challenging environments tend to rely on agents like Tris(2-Methoxyethoxy)vinylsilane. Its value reaches into automotive lines, electronics plants, and construction sites, delivering bonds where failure isn’t an option. Looking at the challenges through human experience, safety training and investment in greener options give both workers and end-users more confidence in the products around them. Every time technology leaps forward, it’s worth asking how raw materials perform—not only for today, but for the future of industry and the planet.
Tris(2-Methoxyethoxy)vinylsilane goes by the Chemical Abstracts Service (CAS) number 1067-53-4. That string of numbers does more than just label a bottle. Lab workers, safety officers, and quality managers worldwide use this number to identify the compound unambiguously. Decades ago, before digitization, mix-ups happened all the time—a missed digit in a chemical name, a translation error, and research could slide off the rails. With unique identifiers, that confusion just doesn’t fly anymore.
Anyone who’s worked with chemicals knows label clarity affects everything. A CAS number provides instant certainty. Walking in a storeroom and spotting “Tris(2-Methoxyethoxy)vinylsilane” with CAS 1067-53-4 lets a chemist scan databases, pull up toxicity data, find the right safety sheet, and avoid accidents. Years back, a research team at my university ordered a chemical by its trade name. It arrived, had a close-sounding generic name, but the properties didn’t line up. Delayed experiments and wasted money followed. If we’d used the CAS number, we could’ve avoided trouble.
Supply and demand in manufacturing runs on precision. Businesses source raw materials across borders and languages; a numerical label like CAS 1067-53-4 helps everyone keep track. Distributors won’t mix up your order. Regulators want the right paperwork every time a drum crosses a port. The right CAS number weeds out fakes. About twenty percent of regulatory interventions worldwide stem from mistaken or misidentified chemicals, according to a report by the European Chemicals Agency. That’s a number no one should ignore, especially anyone responsible for QC or compliance.
Risk isn’t theoretical. The CAS number makes sure that every researcher, from a grad student to a plant manager, pulls the correct safety information. Tris(2-Methoxyethoxy)vinylsilane’s safety characteristics tie directly to its CAS number, so database searches pull relevant protocols for handling, disposal, and transport. In my line of work, even experienced techs sometimes grab the wrong material because packaging looked similar. Getting the right number—and using it every time—cuts out guesswork.
The chemical industry still faces problems with outdated labels or unclear documentation. Small labs, research startups, and even high schools can slip up and use informal names or company-specific codes. Updating digital inventory systems, displaying the CAS number front and center on product sheets and containers, and training staff to use CAS numbers as a search ‘passport’ shrinks those knowledge gaps.
Suppliers who communicate clearly with the CAS number, buyers who crosscheck that number in contract paperwork, and researchers who log results using it—each builds a web of trust. From personal experience, these habits empower teams to share findings openly and speed up progress. Regulatory, clinical, and academic communities have pushed for standardization for good reason. Without it, errors multiply and safety nets fray.
Getting comfortable with CAS numbers doesn’t just follow a rule; it avoids headaches and protects people. Digital systems only work if you feed them trusted data. Cross-referencing that number in public and commercial databases matters. Keeping up with updates from the Chemical Abstracts Service should be part of every lab’s SOP. The more everyone treats the CAS number—like 1067-53-4 for Tris(2-Methoxyethoxy)vinylsilane—as the common language, the less risk finds its way through the cracks.
Anyone handling specialty chemicals like Tris(2-Methoxyethoxy)Vinylsilane knows good habits at the storage stage can mean the difference between a smooth operation and a worrying incident. This isn't something to treat lightly. The clear, slightly viscous liquid can react with moisture, air, or heat, and nobody wants to clean up after a chemical reaction that could've been avoided. Many manufacturers and labs rely on its effectiveness as a coupling agent for improving the durability of sealants and coatings in demanding applications, but things quickly go south if you let sloppy storage create a hazard.
One thing I've learned over the years: exposure to moisture invites trouble with organosilanes. Tris(2-Methoxyethoxy)Vinylsilane isn't any different. Any contact with water, even in the air, leads to hydrolysis, where the chemical breaks down and releases methanol—a flammable and potentially toxic substance. The result is not only wasted product but an accident waiting to happen. Storing it in a tightly sealed container in a dry and well-ventilated spot keeps both product and people safer.
I've seen some labs trust makeshift containers or repurpose glass bottles from less sensitive reagents. That has often led to sticky messes and headaches for the safety manager. Fresh, labeled, airtight containers made from materials designed to handle silane-based chemicals cut down on risk and confusion. Stainless steel or certain plastics do a better job at holding up over time compared to cheaper options.
Heat and sunlight speed up chemical reactions. Both degrade Tris(2-Methoxyethoxy)Vinylsilane and create fumes that no one wants in an enclosed space. In my last job, a single bottle got left on a cart under direct sunlight for less than an hour. The pressure inside the container climbed fast, even in a short time. Placing the stock in a shaded storage area with air conditioning, away from radiators or windows, solves this. Don't skip reading the supplier's paperwork on temperature recommendations—a good rule of thumb keeps it below 30°C (86°F). Some labs even take it one step further and use dedicated chemical refrigerators, especially if bulk supply sits for months.
It’s easy to overlook what's sitting near each other on the shelves. Tris(2-Methoxyethoxy)Vinylsilane can react if it leaks or its vapors come into contact with acids or oxidizing materials. I learned the hard way that storing it beside strong acids led to corroded shelving. Group it with other compatible organosilanes, and keep acids, peroxides, and strong oxidizers far away.
Inventory checks every few weeks make a huge difference. Safety data sheets point out changes in color or cloudiness as warning signs. If the liquid looks off, don't risk using it; arrange for professional disposal.
I've seen more confusion and close calls from unlabeled bottles than from any other mistake in chemical storage. Printed labels—firmly attached—give at-a-glance info on hazards, storage guidelines, and what to do in an emergency. Pair this with regular safety briefings, especially for newer staff, and you're well ahead of most common issues.
Good storage isn't fancy or expensive. It just comes from consistency and care—habits that protect not only the product but everyone who works nearby.
Tris(2-Methoxyethoxy)vinylsilane comes up in the chemical industry as a silane coupling agent, linking inorganic and organic materials. You’ll spot it in adhesives, sealants, paints, plastic compounding, and sometimes wire insulation. It improves flexibility and strength, and chemists appreciate its moisture resistance benefits. People working in labs and plants interact with this compound more than everyday folks, but products treated with it can sit in homes and workplaces across the world.
Looking up safety resources like the European Chemicals Agency and the Globally Harmonized System, this silane checks some hazard boxes. Studies flag concerns about eye and skin irritation. If someone spills it on their arm or hand, they’ll likely end up with a rash or redness before long. Dust and vapor can bother the lungs, leading to coughing or scratchy throat. One study highlighted moderate toxicity in rodents at higher exposures. Long-term data on chronic effects are limited, but early signs point more to acute reactions than hidden dangers lurking years down the line.
I remember a chemical handling training where the instructor stressed never letting silane-based products touch bare skin or eyes. Colleagues who didn’t take that to heart got stung with strong irritation—enough for them to never cut corners again. It can seem like routine work, but direct contact feels anything but routine.
Unlike outlawed toxic substances such as asbestos or lead, Tris(2-Methoxyethoxy)vinylsilane hasn’t triggered sweeping bans. It’s not a known carcinogen or reproductive toxin under major regulatory systems. Still, just because something isn’t classified as highly toxic doesn’t mean it’s risk-free. Overspray, splashing, or inhaling vapors create real problems if someone gets careless.
OSHA and similar agencies ask for gloves, goggles, and good ventilation when handling this compound. Big-name chemical producers echo these recommendations, highlighting the need to use containment and local exhaust.
Real protection starts with reading the Safety Data Sheet before the first use. After years working around hazardous chemicals, reading the fine print always paid off. Safe habits make a huge difference—always wearing gloves, goggles, and tested respirators. Wash hands and arms after handling, and don’t eat or drink in the same area. Only work in well-ventilated spots or under fume hoods when mixing or applying liquid silanes.
Employers must train staff, post safety signage, and check that equipment like eyewash stations works. Regular risk checks help spot where things might go wrong: leaks, open containers, or improper storage. Prompt cleanup of spills with proven absorbents keeps floors slip-free and limits evaporation.
Green chemistry efforts hunt for safer replacements. Some labs test silane-free additives with fewer irritant effects, but chemistry doesn’t always cooperate without trade-offs in performance. Until breakthrough options hit the market, safe handling remains the core strategy. Transparency in supply chain practices helps too, as buyers can push for better formulations when safety concerns reach the right ears.
Anyone dealing with Tris(2-Methoxyethoxy)vinylsilane needs to respect its hazards, even if it doesn’t make headlines for chronic toxicity. With the right precautions, industry professionals keep risk low. Sharing real-world stories and facts helps everyone stay sharp and safe—far better than learning the hard way.
Sometimes, a chemical name like Tris(2-Methoxyethoxy)Vinylsilane seems intimidating, but breaking it down brings a lot more clarity than might be expected. The chemical formula, C11H24O6Si, covers the entire structure of this silane: three 2-methoxyethoxy groups bound to a silicon atom, plus a vinyl group. In the chemistry community, pinning down the correct formula is non-negotiable. Every atom holds significance, especially in industries where this compound plays a part.
Tough composites, adhesives that don’t peel, and coatings that last through some harsh weather often depend on chemicals like this silane. Each methoxyethoxy group helps it mix with a wider range of materials. The vinyl group enables it to bond tightly when heat or catalysts get involved. The formula C11H24O6Si isn’t just a detail for the lab—it marks out what this molecule can actually do, how it reacts, and how it’s handled safely.
Anyone manufacturing electronics or formulating adhesives learns that skipping over chemical detail can cost plenty down the road. Using the right silane, with the right structure, means fewer product failures and stronger materials. In my own work, switching to compounds with clear, reliable formulas cut down lab trial time and gave us more consistent results in applications ranging from paints to fiber optics. Even suppliers and warehouse managers have to know their chemicals, right down to the formula, because guidelines and storage can change based on the exact makeup.
Stories in industry circles show what happens with a mislabeled drum—a minor formula difference between two silanes led to thousands in ruined stock. An off-label mistake may seem tiny in the paperwork but makes a big mess later, from workplace safety fines to legal headaches. The chemical formula serves as a final checkpoint for quality control, regulatory compliance, and safe handling. Tris(2-Methoxyethoxy)Vinylsilane, with its C11H24O6Si structure, has its own set of hazards and shelf life, only matching the official safety sheets if the formula stays accurate.
Chemicals don’t forgive shortcuts. Accurate chemical formulas need double checks at every stage—from the purchase order, labels, and invoices right through to the mixing line. Training staff to read and recognize formulas, not just product codes, builds in another layer of safety. Tighter systems around documentation reduce missteps. These solid practices lean on simple facts: chemistry is as strong or as fragile as the weakest link in how we track, store, and use each compound. It’s not just about science but professionalism, too.
Trust builds through solid facts, experienced advice, and up-front documentation. For those who spend time in a lab or work with materials that end up in consumer goods, this trust in chemistry’s details turns into everyday safety, satisfied clients, and a lower stress load. A formula like C11H24O6Si guides purchasing, research, risk assessments, and even insurance. The story of Tris(2-Methoxyethoxy)Vinylsilane reminds everyone: details matter, and real progress moves forward one clear, trusted step at a time.
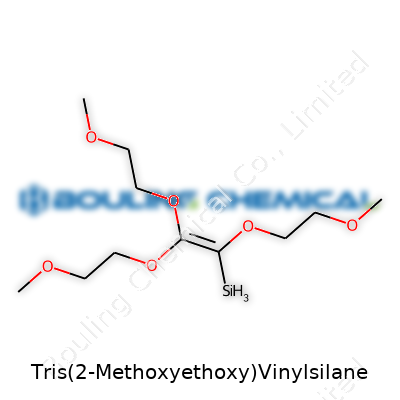
| Names | |
| Preferred IUPAC name | Tris[2-(methoxyethoxy)ethyl]ethenylsilane |
| Other names |
Vinyltris(2-methoxyethoxy)silane Tris(2-methoxyethoxy)vinylsilane Vinyltri(2-methoxyethoxy)silane Silane, tris(2-methoxyethoxy)vinyl- A-172 Dow Corning Z-6032 |
| Pronunciation | /ˈtrɪsˌtuːˌmɛθɒksiˌiːθɒksiˌvɪnɪlsɪˈleɪn/ |
| Identifiers | |
| CAS Number | 1112-39-6 |
| Beilstein Reference | 2298736 |
| ChEBI | CHEBI:87643 |
| ChEMBL | CHEMBL570856 |
| ChemSpider | 18259549 |
| DrugBank | DB13840 |
| ECHA InfoCard | 100.194.293 |
| EC Number | 220-941-2 |
| Gmelin Reference | 107157 |
| KEGG | C18715 |
| MeSH | C017693 |
| PubChem CID | 6913996 |
| RTECS number | YN9100000 |
| UNII | 0D37ABP674 |
| UN number | UN1993 |
| CompTox Dashboard (EPA) | DTXSID8031804 |
| Properties | |
| Chemical formula | C11H24O5Si |
| Molar mass | 250.39 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Characteristic |
| Density | 1.02 g/mL at 25 °C (lit.) |
| Solubility in water | Insoluble |
| log P | 0.6 |
| Vapor pressure | <0.5 mmHg (20 °C) |
| Acidity (pKa) | 12.3 |
| Basicity (pKb) | pKb ≈ 6.5 |
| Magnetic susceptibility (χ) | -6.55·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.427 |
| Viscosity | 5 mPa.s (25°C) |
| Dipole moment | 4.61 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 541.3 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | V03AB37 |
| Hazards | |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | Precautionary statements: "P261, P264, P271, P272, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P333+P313, P337+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 93 °C |
| Autoignition temperature | 250 °C (482 °F) |
| Lethal dose or concentration | LD50 (oral, rat): > 2,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral Rat 10,670 mg/kg |
| NIOSH | PY9944 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 50 ppm |
| Related compounds | |
| Related compounds |
Trimethoxyvinylsilane Triethoxyvinylsilane Vinyltriacetoxysilane Vinyltrimethoxysilane Vinyltriethoxysilane Methacryloxypropyltrimethoxysilane |