Tripiperazine dicitrate carries a backstory that links pharmaceutical progress and the wild arc of scientific curiosity. In the middle of the last century, chemists searched for molecules that could interrupt certain nerve pathways and stumbled across piperazine derivatives. This particular salt, formed from tripiperazine and citric acid, answered the growing demand for antihelmintic agents in human and veterinary medicine. Big names in Eastern and later Western pharma drew up patents, trying different routes to optimize its stability and solubility. Laboratories once focused on piperazine’s simpler forms but soon figured out that tweaking with citrate elevated its usefulness, especially when large-scale synthesis got involved. The connection with safer, more soluble anti-parasitics kept it in focus, especially through the 1970s and 1980s. Looking at its path through regulatory environments, this molecule had its ups and downs—its place in essential medicines lists, off-label uses, and reinvention for research models all tell the story of a molecule shaped by real needs in hospitals, fields, and research labs.
Tripiperazine dicitrate can take on several forms: fine white powder, granular solid, sometimes packed as a crystalline substance for better handling in humid climates. Makers usually focus on the salt form because it mixes well with water and holds up during sort of rough handling typical in field environments. It plays its biggest role as an anti-parasitic, but research teams value its chemical backbone for synthesis work. Most packaging information highlights its water solubility, ease of measurement, and shelf life at moderate temperatures. For me, packing stability and the avoidance of moisture exposure have always been core issues; lose either, and you’re looking at quick degradation and a hit to expected potency.
This compound shows off several traits that matter in lab and field work. Water solubility stands out: at about 10 grams per 100 ml at room temperature, anyone working with aqueous solutions gets straightforward handling. Its melting point usually lands in the 110–140°C range, a useful element for processing settings that might face temperature spikes. The scent? Basically none, a relief for anyone used to acrid chemical reagents. The molecule weighs in at 621.7 g/mol, which fits into the typical profiles for salt-based antihelmintics. Tripiperazine dicitrate does not change color in air over days, so you can spot batch problems with a quick look. Chemically, the molecule gets attention because its three piperazine rings link through strong nitrogen bonds, giving unique reactivity that researchers sometimes leverage to attach new groups.
On any bottle or packet, you’ll notice clear CAS and EC numbers. Batches commonly arrive with purity above 98%, as lower levels hit both clinical outcomes and lab repeatability. Labels list exact salt content, water percentage, production date, and best-by dates set in months. Documentation seems dense, but those details anchor safe handling and dosing. In my own experience, technical sheets highlighting heavy metals, organic residue, and pH allow researchers to trust their results. The best producers always include origin, necessary hazard notes, and recommended storage conditions, ticking off safety rules set by global and national agencies.
Manufacturers usually synthesize tripiperazine first by using strong base-catalyzed cyclization of ethylene diamine derivatives with dichlorodiethane, then use precise stoichiometry to balance piperazine and citric acid for salt formation. This part of the process needs attention; get proportions wrong or botch the temperature and the resulting batch fails purity analysis. Modern reactors set up closed systems for safety and batch consistency. Precipitation under cold conditions lets crystals separate neatly without excess water, which keeps downstream steps consistent. Drying at controlled temperatures and sieving become final quality checks before packing. Sometimes the procedure includes activated carbon to purify and knock out unwanted organics. Each prep seems simple on paper but needs sharp monitoring: under-reacted starting materials tend to linger as contaminants, posing risks in both direct applications and further syntheses.
Tripiperazine dicitrate holds promise for chemists looking to change or expand its core structure. The multiple nitrogen atoms across its rings act as nucleophiles, which helps for N-alkylation or acylation reactions. I’ve seen graduate students use the compound as a base for harder-to-synthesize anti-parasitic candidates. The citrate portion sometimes undergoes hydrolysis under basic conditions, providing a window to experiment with salt forms. Mild oxidizers react with the piperazine ring, breaking it to form open-chained diamines. Research teams sometimes explore attaching metal ions, aiming for higher potency or selective delivery in biological systems. Real-world work here always faces yield and selectivity problems, especially when pushing for scale, but those who control their purification steps walk away with high-value intermediates.
Common synonyms include “3,3’,3’’-Triazinane-1,4,7-triyl triamine citrate” and “Tris(piperazin-1-yl)methane dicitrate.” Trade names pop up mainly in veterinary supply catalogues, though in some countries you’ll spot heavily localized names, reflecting their regulatory labeling practices. Some distributors lean on “Triazinane citrate,” favoring simplicity. Researchers must check local lists to avoid confusion, as regulatory filings sometimes list older synonyms or legacy product identifiers. Whenever I worked across countries, sorting by synonym usually made or broke audit trails for raw material flows—aligning sample codes and trade names matters more than it seems at first glance.
In the lab, handling tripiperazine dicitrate never feels casual. The compound carries clear hazard signals: skin and eye irritation top the list, along with mild inhalation toxicity if dust escapes containment. Safety data sheets advise nitrile or latex gloves, eye shields, and use in well-ventilated hoods. GHS standards align closely with local regulations in most producing countries, requiring spill protocols and first aid equipment in reach. Disposal follows standard routes for basic organic salts, though wastewater treatment considers the molecule’s moderate aquatic toxicity. Accidents almost always stem from dust or splash exposure, so keeping everything dry and sealed makes all the difference. Manufacturing settings audit their processes with a set rhythm, following occupational health standards and enforcing regular PPE checks. Controlled inventories and batch tracking prevent accidents linked to mislabeling or misstorage, something I’ve seen drive up insurance claims and compliance headaches in more than one facility.
The major use for tripiperazine dicitrate remains in anthelmintic therapy. Both human and animal dosages target a broad spectrum of parasitic worms, offering reliable results with limited cross-reaction in the gut. Rural health programs often list it as a staple for school-based deworming, thanks to its oral dosing and tolerable taste profile. In veterinary work, it lines up as a frontline treatment for roundworms in livestock, keeping food safety standards high for downstream meat and dairy production. Away from clinics and barns, the pharmaceutical research field leans on tripiperazine’s stable backbone to engineer new scaffolds for CNS drugs and ion channel inhibitors. Some studies test the molecule’s capacity to deliver controlled release when combined with hydrogels, expanding its appeal into slow-acting formulations. In other settings, it appears as a reference standard for chromatographic calibrations, anchoring quantification where accuracy means everything to compliance and safety.
Universities and private firms look at tripiperazine dicitrate with eyes set on improving anti-parasitic activity, tweaking the molecule’s structure through rational drug design. Recent research goes further, studying its interactions with ion channels, offering clues for future drug development in neurology and cardiology. A stream of papers covers modifications to enhance absorption in the GI tract, reduce toxicity, and widen the effectiveness spectrum. R&D budgets funnel toward high-throughput screening, machine learning-guided analog design, and green chemistry approaches to trim waste and energy during synthesis. Partnerships between agritech companies and national labs feed studies into soil and water fate, vital for ruling out environmental buildup or off-target effects. These efforts are not just academic; they shape access for poor communities, push safety limits higher, and branch the compound’s potential into new disease areas.
Toxicity profiles for tripiperazine dicitrate balance animal studies, cell culture work, and epidemiological tracking for treated populations. Acute toxicity tends toward gastrointestinal distress at high oral doses, with rare severe systemic reactions. Chronic effects show up only at sustained high levels, usually outside therapeutic ranges, but oversight committees watch for subtle neurotoxic or reproductive effects, urged by data from long-term animal experiments. In the environment, tripiperazine derivatives can harm aquatic invertebrates, a real point of concern for large-area use in agriculture. Regulatory agencies set maximum residue limits in food and water, relying on a steady flow of new data to keep standards grounded in current evidence. Toxicology research adopts new in silico models and metabolite tracking, tested side-by-side with in vivo studies to close data gaps. My experience with regulatory audits taught me the importance of rapid response to new findings—one flagged abnormality can halt shipments or spike public worry almost overnight.
Tripiperazine dicitrate’s future draws power from two directions: technology pivots in drug delivery and rising interest in sustainable agriculture. Pharma companies keep an eye on nanoparticle encapsulation and controlled-release platforms that might improve effectiveness and slash side effects, especially in regions battling drug resistance. Crop science teams want clearer data on the compound’s breakdown in different soils, mapping risk and opportunity in one file. Across regions hit hardest by parasitic disease, governments invest in updating distribution to keep stocks available and affordable, blending old molecules into new public health campaigns. Machine learning in molecule design could spin off analogs with tailored biological targets, opening doors into new types of medicine. The sum of all this—more data, faster modification cycles, and wider collaboration—drives the product’s story into new labs and clinics, keeping its role in disease control and research alive longer than most imagined when it first entered the pharmacopeia.
Tripiperazine dicitrate sticks out in pharmacology for its use in treating conditions involving parasites in the human gut. It's a compound developed to tackle worms—specifically, intestinal roundworms and pinworms that often infect kids and sometimes adults. Growing up in a crowded part of town meant that I saw many friends deal with these stubborn infections. The reality is, places without enough clean water or regular handwashing struggle most with these parasites. Dealing with the itchiness and stomach upset can distract kids from school and keep adults from work. Medicines like tripiperazine dicitrate help put life back on track.
This compound acts by interrupting nerve signals in worms, forcing them out of the body. Unlike some treatments that try to poison or break down parasites, tripiperazine paralyzes them. Worms can't hold on inside the intestine, so the body flushes them out naturally. That sort of targeted approach gives people relief without dumping excessive chemicals into the system. The process is especially gentle for young children, who often struggle with more aggressive medications.
Many overlook the importance of using these medications correctly. Overuse or improper use can help create resistance, just like with antibiotics. I remember a local health campaign emphasizing the need to finish every dose, even when symptoms faded. Too many families tossed away leftover medicine after two days, only to see symptoms come roaring back. The worms had never really left. Fact is, a single round of treatment often isn't enough when reinfection risks stay high.
Tripiperazine dicitrate only helps for certain worms; it won’t clear out all parasites or bacteria causing gut trouble. Relying on old advice or grabbing whatever is available at a corner pharmacy doesn’t work. Qualified healthcare professionals rely on stool tests or symptom histories before picking the right medicine. Common side effects of piperazine treatments include nausea, stomach cramps, dizziness, and occasionally allergic reactions. Some conditions, such as kidney or liver problems, call for alternatives because the medicine can stress these organs. My uncle, who suffered from chronic kidney disease, always needed a doctor’s discretion before trying any anti-parasitic drugs.
Worm medicine works best alongside clean living environments. I’ve watched community teams dig new latrines and teach local kids to wash hands before meals. Medical outreach programs offering tripiperazine dicitrate alongside hygiene classes delivered the strongest long-term results. Without these combined efforts, reinfections often spike. Access to clean water, regular education campaigns, and consistent drug supply all play a role in reducing health problems linked to intestinal worms.
Addressing worm infections requires a layered approach: medicine, hygiene, and public awareness. Local leaders succeed by drawing from medical evidence and lived experience. Pharmaceutical quality checks ensure that products reach pharmacies in good condition. Continued investment in education for families, teachers, and health workers reduces the burden on emergency care down the line. In my experience, one effective change meant handing out soap along with medicine. Small actions multiply when a community rallies together. Tripiperazine dicitrate forms just one piece of a bigger puzzle in preventing and treating harmful worm infections.
Tripiperazine dicitrate isn’t a medication doctors reach for lightly. Most folks taking it are treating specific conditions like certain kinds of parasites or very persistent itching. With any medication, side effects can crop up and make life more complicated, and this one is no exception.
Many people report digestive problems. Think about nausea that seems to stick around most of the day, sometimes enough to spoil any hopes for a decent meal. Some have taken it and found themselves making extra trips to the bathroom—diarrhea isn’t rare. Stomach cramps and discomfort also appear high on the list.
Some patients explain how they started feeing unusually drowsy or dizzy not long after their dose. The world can take on a fuzzy edge, which makes driving or doing anything requiring sharp attention risky. Others talk about a dry mouth that refuses to go away, no matter how much water they drink. This can be especially annoying, and sometimes it shows up alongside mild headaches.
Skin rashes have cropped up in a handful of people. Itchy welts or red patches can appear with little warning, which can make daily tasks uncomfortable or embarrassing, especially around co-workers or classmates.
Some cases get trickier. Serious allergic reactions—swelling, trouble breathing, or hives—can’t be ignored. Friends or family who see someone’s lips or face puffing up or see them gasping for air should call for medical help right away. Reports of jaundice—yellowing eyes or skin—forces a rethink unless there’s a solid reason to continue the medication.
Tripiperazine dicitrate can also impact the liver. Liver test numbers can go up, and that’s bad news if ignored. Those with a history of liver issues have to stay in close touch with their doctor. Seizures are rare but possible, especially in people with other neurological conditions.
Doctors want to track how people respond to this drug for a reason. The side effects can overlap with symptoms of other problems, confusing both patients and health workers. Only a qualified doctor or pharmacist can separate drug issues from usual illnesses.
Some folks ask if the side effects wear off over time. For some, yes, but for others the discomfort lingers or gets worse. Stopping the medicine without help can create its own set of problems, so no one should decide to quit or change the dose on their own.
Small, frequent meals may help with nausea and stomach upsets. Staying hydrated can combat dry mouth and mild headaches. Anyone on this medicine should keep a note of their symptoms in a diary, as it helps doctors decide if something needs to change.
Don’t hide side effects—even ones that seem minor. Honest conversations with healthcare professionals keep people safe. Pharmacists often have tips for managing routine problems like dry mouth or drowsiness, and they can remind patients about what warning signs to watch for.
Tripiperazine dicitrate comes with a real risk for side effects. When it works, it can do its job, but not everyone finds the road smooth. Understanding what can go wrong helps people catch problems before they get serious. And sometimes, switching to a different approach or adding supportive treatments makes a difference.
Doctors often prescribe Tripiperazine Dicitrate to help treat certain types of worm infections. This medication holds an important place in my memory, especially when helping my uncle manage a pinworm infection a few years ago. He struggled with the directions at first, so I learned firsthand how everyday mistakes can happen. Medications like this work best when people take the proper dose at regular intervals, not skipping or doubling up. Swallowing the tablet with a full glass of water and avoiding crushing or chewing usually leads to better results and fewer stomach issues.
Some people forget that eating habits can affect how well this medicine does its job. I remember a physician explaining that taking Tripiperazine Dicitrate after a light meal can lower the chance of nausea. Fatty or heavy meals might upset the stomach. Sticking with easy, bland meals before and after taking each dose makes the experience more pleasant.
The dose can differ based on age, body weight, and infection type. Kids often get a different amount than adults. My neighbor mixed up her son's liquid and tablet dose, creating confusion and very mild side effects like dizziness. She learned the hard way that following printed instructions and asking the pharmacist for clarification could have saved trouble. People who try to “speed up” the process by taking more usually end up feeling worse, so it pays to stick with what the doctor recommends.
Certain health problems call for extra care. I once watched a diabetic friend struggle when she took a new medication without mentioning her blood sugar challenges. Tripiperazine Dicitrate interacts with some drugs and can impact people with liver or kidney conditions. Informing your doctor about all current prescriptions and supplements helps avoid tricky situations. Honesty at the pharmacy can lead to better recommendations and fewer negative surprises.
Even with the best intentions, side effects can pop up. Nausea, vomiting, or unusual tiredness shouldn’t get brushed off. My aunt noticed skin rashes after using a deworming pill, so she called her healthcare provider promptly. Quick action often prevents simple problems from growing serious. Keeping the medicine in its original packaging and away from curious pets or children adds a layer of safety at home.
Internet forums and secondhand stories cause plenty of confusion around unfamiliar medications. The Food and Drug Administration and reputable health websites provide honest and accurate facts. Reading the leaflet that comes in the box sounds simple but gives unmatched insights into warning signs and drug interactions. Pharmacists know more than many think—chatting with them has saved my family members time and stress during prescription pick-ups.
Tripiperazine Dicitrate delivers solid results when used with care, attention, and open communication with healthcare professionals. Most problems come from simple misunderstandings or skipping directions. Choosing a routine, staying informed, and reaching out for advice create an environment where medicine can work its best.
Tripiperazine Dicitrate sits among lesser-known pharmaceutical names. It gets prescribed for specific health scenarios, mainly for its anti-parasitic properties. In regions where intestinal worms cause problems, this drug occasionally shows up in the discussion. Sometimes people think of medications as one-size-fits-all solutions—but behind every pill, there’s a set of realities about side effects and contraindications. What matters most is understanding who shouldn’t touch this drug and why, based on documented evidence, medical experience, and practical wisdom.
Not every medicine fits every patient. Tripiperazine Dicitrate brings its list of restrictions like many other synthetic compounds. Individuals who show hypersensitivity to piperazine drugs need careful screening before any prescription. Allergic reactions can range from mild rashes to severe anaphylaxis. Once you see a pattern of rashes, hives, swelling, or breathing trouble after taking a drug in this family, another round risks stronger responses. Ignoring these warnings places the patient in harm's way.
People living with chronic kidney disease run a higher risk from this medication. Healthy kidneys help clear tripiperazine metabolites, but in renal impairment, these compounds linger longer, raising toxicity risk. Symptoms include muscle tremors, weakness, and even convulsions. Most clinicians stay clear of piperazine drugs when kidney function drops below safe margins.
Children under the age of two hold their own set of risks. The nervous system in babies and toddlers develops rapidly, and substances interfering with neural signals can trigger unexpected reactions. Tripiperazine has been associated with neurological side effects, including seizures. Pediatricians prefer alternative treatments for worms in this age group.
Pregnancy and breastfeeding present uncertainties. Studies on piperazine safety before and after birth remain limited. With animal data suggesting possible harm and not enough solid human evidence, best practice means seeking alternatives when treating pregnant or breastfeeding patients. If worm infection becomes a threat to maternal health, weighing the danger of untreated illness against drug risks requires thoughtful doctor-patient discussion.
My time spent in clinics taught me one thing: rare side effects turn up more often than textbooks admit. Tripiperazine Dicitrate carries the same burden. Some adults have experienced confusion or vision problems after use—side effects that never get fair mention in quick consultations. In communities with limited access to allergy and metabolic screening, missing these reactions is all too easy. Families sometimes self-medicate using leftover deworming pills, which only compounds the danger without professional oversight.
Patients and providers both need clearer information. Medication labels deserve plain language about kidney safety, allergy risk, and age restrictions. Quick reference guides in clinics could save trouble by reminding staff which symptoms spell trouble. Repeating the message at every level—doctor’s office, pharmacy counter, public health campaigns—helps reinforce the point.
Doctors benefit from continuing medical education that covers less-commonly used drugs. Patient records can include allergy alerts and renal function histories before unknown medications reach the bedside. Rural clinics and community health workers play an outsized role in safely delivering treatment where sophisticated labs don’t exist, by sticking to established safety checklists.
The bottom line: awareness keeps people safer. Medicines like Tripiperazine Dicitrate may serve their purpose, but not everyone meets the profile. Asking questions, sharing medical history, and sticking with trusted sources builds the shield that patients deserve.
Tripiperazine dicitrate isn’t a name that pops up in everyday conversation. This compound works in the body as an antihistamine and sometimes as an antiemetic. You might see doctors prescribe it for allergy symptoms or to settle the stomach. Still, most people who have needed medication while pregnant or breastfeeding know a simple truth: there’s never a risk-free answer.
Pregnancy always leads to questions about what’s safe. A lot of medications have not been fully studied in pregnant people, and tripiperazine dicitrate fits this pattern. Science hasn’t provided enough high-quality research to say for sure if this medication can hurt a developing baby or show up in breast milk. Sometimes it’s tempting to take comfort in years of clinical use, thinking older drugs must be safe. The reality: just because something's been used for a while doesn’t mean we have all the answers.
Pregnant and nursing patients deserve advice rooted in real science and long-term data. Pharmacists and doctors agree that safety in pregnancy comes down to weighing benefits against risks. If symptoms like severe nausea or allergies become overwhelming, there’s sometimes a reason to consider a medication. For tripiperazine dicitrate, professional organizations such as the American College of Obstetricians and Gynecologists have not listed it among first-choice medications in pregnancy or while breastfeeding. With limited evidence, most doctors usually point to better-studied alternatives.
As a parent, it’s all too easy to scan the internet for quick reassurance. There are lots of anecdotal stories online but a real gap in expert-controlled research. No one wants to be a test case, especially when it comes to a developing baby. Taking a medicine with unknown risks can lead to anxiety that doesn’t go away. Doctors look for riskier side effects, and with tripiperazine, there’s a chance of drowsiness, dry mouth, or movement changes that can also affect infants.
Many medications prescribed during pregnancy and lactation get used based on legacy practice rather than on firm evidence. Companies focus on diseases that affect large populations, so drugs for milder symptoms during pregnancy get less attention. Pregnant or breastfeeding individuals need research designed for them—studies that clearly outline what crosses the placenta or enters milk, what’s safe, and what’s not. This gap shines a light on a widespread problem in medicine, not just with tripiperazine dicitrate but in many other cases where guidance relies on best guesses, not rigorous facts.
If you’re facing tough allergy or nausea symptoms during pregnancy or breastfeeding, involve your care provider right away. Bring specific questions to the table: What’s the evidence for this drug in pregnancy? Are there safer or non-drug ways to manage these symptoms? Sometimes, careful timing of doses or choosing alternatives already proven safer can help. For breastfeeding, doctors check if the medication might pass into milk and what that might mean for the infant. Shared decision-making and honest conversations make a big difference.
The bigger story here is about protecting the next generation and supporting informed choices. Patients want and deserve clearer answers. Doctors want stronger research and guidelines. For now, caution and careful discussion with healthcare professionals offer the best path forward, especially with drugs like tripiperazine dicitrate that haven’t yet earned clear safety marks for pregnancy or breastfeeding.
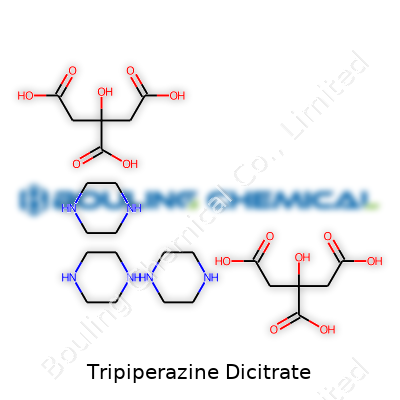
| Names | |
| Preferred IUPAC name | 1,4,7-Triazacyclononane trihydrocitrate |
| Other names |
2,3-Piperazinediacetic acid 4,5-bis(1,1-dimethylethyl) ester Piperazine citrate 1,4-Diazacyclohexane citrate |
| Pronunciation | /trɪˌpaɪ.pəˈreɪ.zin daɪˈsɪt.reɪt/ |
| Identifiers | |
| CAS Number | “14402-74-7” |
| 3D model (JSmol) | `"3D model (JSmol)": "3Dmol.js?model=O.C(CCN1CCN(CC1)CCN2CCN(CC2)CCO)C(=O)C(O)=O.C(CC(=O)O)(C(=O)O)O"` |
| Beilstein Reference | 3521817 |
| ChEBI | CHEBI:9458 |
| ChEMBL | CHEMBL2105207 |
| ChemSpider | 608039 |
| DrugBank | DB14657 |
| ECHA InfoCard | ECHA InfoCard: 100.192.302 |
| EC Number | EC 241-372-9 |
| Gmelin Reference | 113301 |
| KEGG | C18633 |
| MeSH | D014273 |
| PubChem CID | 124327204 |
| RTECS number | TP2080000 |
| UNII | 2Z60B35P6E |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C18H39N9O14 |
| Molar mass | 966.99 g/mol |
| Appearance | White or almost white crystalline powder |
| Odor | Odorless |
| Density | 1.29 g/cm3 |
| Solubility in water | Soluble in water |
| log P | -3.2 |
| Acidity (pKa) | pKa ≈ 9.8 |
| Basicity (pKb) | 2.98 |
| Refractive index (nD) | 1.52 |
| Dipole moment | 4.49 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 292 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | ΔfH⦵298 [Tripiperazine Dicitrate] = -2585 kJ/mol |
| Pharmacology | |
| ATC code | N05AB53 |
| Hazards | |
| Main hazards | Harmful if swallowed; may cause irritation to the skin, eyes, and respiratory tract. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | No hazard statements. |
| Precautionary statements | P264, P270, P273, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Lethal dose or concentration | LD50 (rat, oral): 370 mg/kg |
| LD50 (median dose) | LD50 (median dose): Mouse oral 6600 mg/kg |
| NIOSH | Not established |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 300 mg daily |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Piperazine Dipiperazine Citrate Tripiperazine Hexahydrate N-Methylpiperazine Tripiperazine Tetrahydrochloride |