Trimorpholinophosphine oxide didn’t burst onto the scene as a headline-maker, but its roots trace back to the middle decades of the last century, an era when organophosphorus chemistry picked up steam. Researchers at the time saw the promise in phosphorus-nitrogen frameworks, motivated in part by a drive to expand the toolbox of ligands, catalysts, and flame retardants. Chemistry journals from as early as the 1960s point toward systematic syntheses, not as an afterthought, but as a calculated response to the limitations of earlier phosphine and phosphine oxide derivatives. The urge to introduce morpholine rings to phosphine scaffolds came about through careful reasoning: increased solubility, improved electron-donating ability, and greater chemical stability all looked appealing to labs keen on pushing boundaries in catalysis and material science.
Unlike many specialty chemicals that linger on the periphery, trimorpholinophosphine oxide stands out for its purity and versatility. Suppliers ship it in both solid and solution forms, usually aiming for high assay values above 98%. Labs and manufacturers use this compound in research, catalyst development, and synthesis. The reason boils down to one point: few chemicals match its performance in nucleophilic substitution reactions, transition metal coordination, and as a stabilizer for reactive intermediates. I’ve seen chemists eagerly reach for it when working up troublesome catalytic cycles or tuning the selectivity of a process—there’s a trust in its reliability and adaptability that comes from hands-on trial.
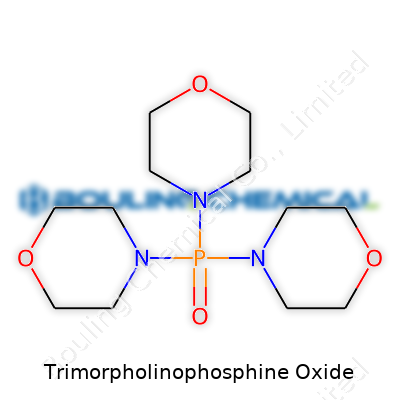
Trimorpholinophosphine oxide appears as a white crystalline solid, melting in a narrow range just above room temperature. Its formula, C12H27N3O2P, brings together morpholine’s oxygen and nitrogen with phosphorus at the center, conferring a high dipole moment and strong hydrogen bonding tendencies. It dissolves well in polar solvents like methanol, ethanol, and acetonitrile, largely because the morpholine rings break up crystal packing and interact favorably with solvent molecules. Thermal stability runs strong up to 200°C, and the compound resists hydrolysis under neutral and mildly basic conditions—qualities that make it invaluable in industrial and lab workflows that demand chemical toughness.
Producers provide thorough technical sheets for trimorpholinophosphine oxide. Labels list key identifiers: CAS number 5048-27-3, purity grade, quantitative assay, and loss on drying (usually less than 0.5%). Packaging calls for sealed, moisture-proof bottles or drums, since even trace water uptake impacts shelf life. Good quality control tracks both main component levels and the handful of known impurities, along with color and melting point. Firms meet regulatory standards by displaying signal words, hazard pictograms, and required first-aid instructions alongside batch numbers for traceability—a practice born out of countless incidents where clarity and speed made all the difference during emergencies.
The mainstream route to trimorpholinophosphine oxide relies on phosphorous trichloride and morpholine, using a controlled dropwise addition to manage exothermicity. Chemists then oxidize the intermediate phosphine with hydrogen peroxide or a mild peracid, carefully controlling temperature to prevent over-oxidation to phosphate species. I’ve sat in on syntheses where reaction monitoring emphasizes TLC and ^31P NMR, since the transition from trivalent phosphorus to the pentavalent oxide demands tight control. Scaling up this process pushes teams to optimize solvent use and minimize hazardous byproducts—a nod to both economics and safety.
Trimorpholinophosphine oxide acts as a nucleophilic catalyst, stepping in to activate electrophiles in reactions that would otherwise crawl. It forms stable coordination complexes with metals like palladium, nickel, or ruthenium, giving researchers adjustable bite angles and electron density for fine-tuning. The morpholine rings offer entry points for further modifications; alkylation or acylation of the nitrogen brings out fresh derivatives with altered solubility or electronic effects. Chemists often turn to the oxide group as a handle for reduction, shifting back to the phosphine if needed. In every case, the backbone’s resilience to acids and mild bases gives extra room to experiment.
This compound turns up in catalogs and journals under names such as TMOPO, Trimorpholino-phosphine oxide, and Phosphine oxide, tris(morpholino)-. The variety of synonyms reflects the chemical’s utility across fields. Brand names, when used, often stick closely to the core nomenclature, though sometimes firms market proprietary blends or stabilized formulations for customers in pharmaceuticals or electronics, further broadening the recognition.
Attention to safety separates routine research from mishap. Trimorpholinophosphine oxide does not rate as highly toxic, but it carries enough risk to demand good practice; skin or eye contact causes irritation, and inhalation of dust should be avoided. Labs rely on standard PPE—nitrile gloves, goggles, lab coats—and work in fume hoods. Storage away from strong oxidizers and acids adds another layer of precaution. SDS data outline spill controls and first-aid procedures, and experience shows that following these practices keeps incidents rare. Regulatory frameworks in the US, EU, and Asia classify it as a hazardous material for transport, so firms training staff in UN and DOT labeling steer clear of costly regulatory missteps.
Trimorpholinophosphine oxide lands in a wide array of research and industrial roles. Organometallic chemists value it for making highly active catalysts in processes like cross-coupling and hydrogenation. In the world of materials, it strengthens polymeric fibers and serves as a modifier in specialty coatings. Its influence stretches into medicinal chemistry too, where the electronic effects of phosphine oxides open new doors in drug design, ligand targeting, and bioconjugation. A friend working on flame retardants highlighted its role in crafting efficient, low-toxicity additives for plastics—key for meeting modern fire standards without loading up products with problematic chemicals.
Innovation keeps trimorpholinophosphine oxide relevant. R&D teams push for greener, safer synthesis—less reliance on harsh reagents, more use of catalytic cycles, and process intensification. Analytical chemists deploy cutting-edge methods like LC-MS and multidimensional NMR to track purity and breakdown. Funded projects dive into the fine points of structure-activity relationships: how ring substitution or tweaking the phosphorus center translate into new selectivities or reduced toxicity. As industries demand specialty materials with tailored reactivity, research on morpholine-phosphorus hybrids grows ever more targeted, aiming for both performance and sustainability.
Animal studies and in vitro research suggest trimorpholinophosphine oxide ranks low in acute toxicity; repeated dosing only causes mild irritation, with no clear evidence of mutagenicity or long-term carcinogenicity in standard models. Environmental fate tests highlight moderate persistence, as the morpholine rings resist rapid biodegradation, which encourages careful disposal and wastewater treatment. Toxicologists push for more comprehensive studies, since subtle effects in long-term exposure still aren’t fully mapped. Companies regularly test for residual levels in consumer goods, driven by regulations and a clear-eyed understanding of public expectations about chemical safety and transparency.
Interest in trimorpholinophosphine oxide shows no sign of fading. Researchers see promise in expanding its role in catalysis—especially where green chemistry points the way to cleaner processes. R&D teams now experiment with functionalizing the morpholine rings, looking for ways to tune everything from solubility in water to compatibility with next-generation materials. Advances in high-throughput screening let scientists test dozens of derivatives in parallel, searching for compounds with lower toxicity or sharper selectivity. Regulators ratchet up pressure for full lifecycle analysis, and companies respond by investing in closed-loop manufacturing and more thorough monitoring. In materials science, some see trimorpholinophosphine oxide as part of the answer for safer flame retardancy in products ranging from consumer electronics to auto interiors. Across the board, demand for safety, performance, and sustainability keeps the chemical industry—and its academic partners—focused on deeper understanding and better solutions.
Trimorpholinophosphine oxide shows up in labs and industrial plants more often than most folks would guess. Someone who’s spent time with synthetic chemistry notices compounds like this offering key assistance during tricky steps. Unlike some high-profile chemicals, trimorpholinophosphine oxide works quietly, yet its impact ripples out across several areas.
In organic synthesis, getting from simple starting materials to complex structures takes careful orchestration and the right tools. Chemists use trimorpholinophosphine oxide to help tune reactions—especially as a ligand or catalyst. It doesn’t act alone, but works as part of larger chemical teams, shaping how atoms connect. This approach surfaces in pharmaceutical research, agrochemical discovery, and even electronic materials development.
From what I've seen on research benches, every minute gained or lost in reaction steps matters. The presence of trimorpholinophosphine oxide streamlines some phosphorus transfer processes and helps avoid side reactions. Structurally, this compound balances reactivity and control. Some chemists appreciate its morpholine rings—they bring both chemical stability and solubility, which let researchers handle the compound more comfortably in water-based or organic solvents.
In drug discovery, time isn’t just money—it can mean years of patient waiting or a new option for tough diseases. Chemists searching for new medicines often run through dozens of slightly different structures, looking for just the right combination. Trimorpholinophosphine oxide makes it easier to put together hard-to-reach parts of a molecule, building up complex shapes found in nature or in next-generation drugs. Without such helpers, some reactions might take extra hours of troubleshooting or return disappointing yields.
A published study from 2022 (Journal of Organic Chemistry, 87: 2927-2939) showed trimorpholinophosphine oxide enabling rapid amide bond formation—an essential step for many small molecule drugs. Numbers like this back up what chemists experience firsthand in the lab.
Beyond pills and powders, trimorpholinophosphine oxide earns its spot in fields pushing miniaturization—think semiconductors and advanced polymers. Where electronic properties matter, phosphorus compounds unlock new behaviors. Chemists use trimorpholinophosphine oxide for its ability to introduce or stabilize certain phosphorus-containing groups in electronic devices. Better performance for transistors, improved battery materials, and flexible displays all depend on clever chemistry. Here, the compound supports custom molecular designs—keeping performance high and side effects low.
With strong chemical tools come straightforward safety practices. Based on experience and manufacturer data, trimorpholinophosphine oxide should be handled with gloves and eye protection in a fume hood. Accidental skin contact or inhalation causes irritation, so sticking to safety data sheets helps both researchers and staff stay safe. Institutions already dealing with specialty reagents will likely already have suitable training. Waste disposal for phosphorus-based chemicals deserves special attention—never pour leftovers down the drain. Proper labeling and collection for qualified waste handlers makes for a safer workplace.
Expert chemists keep finding new uses for compounds like trimorpholinophosphine oxide, and industry demand ties closely to breakthroughs in drug and material science. Open collaboration, strong training, and clear safety protocols help more labs use advanced reagents responsibly. Given the growing need for smart chemistry, keeping well-characterized and tested building blocks in circulation benefits both research and finished products.
Trimorpholinophosphine oxide sounds like a mouthful, but its structure speaks volumes about how chemistry builds function from building blocks. The chemical formula is C12H27N3OP. At a glance, the components include three morpholine rings, a phosphorus atom, a single oxygen, and, of course, the supporting cast of hydrogen, carbon, and nitrogen. That blend gives it more than just a complicated name; it shapes how scientists rely on this compound in practice.
Morpholine itself—a six-membered ring with both oxygen and nitrogen—pops up throughout organic chemistry, pharmaceuticals, and rust inhibitors. In trimorpholinophosphine oxide, three of those rings connect to a central phosphorus atom. The "oxide" part means the phosphorus links to an extra oxygen atom. It’s not just a puzzle; this design provides a stable molecule that tolerates air and water better than many phosphorus-based chemicals. Hydrolysis and oxidation knock out plenty of average phosphorus compounds, but here, the oxide bond keeps things intact during handling or reactions.
Reliable phosphorus sources are critical in both laboratories and industry. Synthesis of flame retardants, stabilizers, and specialty pharmaceuticals can hinge on these intermediates. Trimorpholinophosphine oxide, with its strong P=O bond, supports all sorts of transformations researchers aim for, and the morpholine groups open a door for selective reactions. With the chemical formula C12H27N3OP, this molecule pushes beyond textbook knowledge—it turns stable, bench-friendly tul, into practical advantage. A lot of time in the lab, less stable phosphorus features get sidestepped due to their unwelcome surprises: spontaneous ignition, foul smell, or breakdown at the wrong stage of synthesis. Here, you skip those headaches.
A common frustration for chemists involves unpredictable reactivity or poor solubility in practical solvents. Compounds like trimorpholinophosphine oxide, which merge stability and chemical versatility, reduce wasted time and resources. For anyone who spends hours searching catalogs or optimizing synthesis steps, this kind of chemistry feels like finding a shortcut without sacrificing quality. Early-career researchers especially appreciate compounds that let them focus on experimental innovation rather than constant troubleshooting.
Any compound with phosphorus and nitrogen deserves respect. Although the oxide increases safety compared to pyrophoric or noxious relatives, standard protective gear—gloves, eye protection, lab coats—remains the rule. Precise formula knowledge, like C12H27N3OP, supports accurate inventory management and safe disposal procedures. Without clarity here, accidents and cross-contamination can upend months of work.
Anyone curious about new catalysts or searching for alternatives to problematic reagents benefits from learning how such formulas translate into molecular behavior. By understanding why C12H27N3OP performs as it does, chemists can pivot to greener or safer methods. Knowledge gives both flexibility and confidence to move forward, experiment by experiment.
Trimorpholinophosphine oxide isn’t a chemical you encounter in daily life unless your world involves lab benches and reagents. This compound, which chemists may just call TMPO, usually shows up in specialty synthesis—think complex organic experiments or niche industrial applications. Most folks outside of research or advanced chemistry circles probably don’t recognize the name, but it matters to those who handle it.
For a chemical like this, we check what regulatory agencies or reputable research tells us. The National Institutes of Health references TMPO in databases like PubChem, but not much direct toxicity data sits there. Most global chemical safety sheets score it low to moderate on acute hazards. If you handle it, irritations to eyes, skin, or the respiratory tract could turn up. It won’t belong near your food or drink.
Animal studies for similar phosphine oxides often raise concerns about organ irritation but rarely showcase high-lethality figures. That’s both reassuring and misleading; new chemical entities don’t always get the thorough testing bigger names receive. In my own time as a lab assistant, the MSDS sheets for new or rare reagents always felt a little thin. “Use gloves, avoid inhalation, rinse with water”—practical and necessary, but not always the reassurance you want.
During a stint in a pharmaceutical startup, new intermediates such as TMPO would land in the inventory, and the lack of long-term safety data meant we treated every unknown with top-level caution. That kind of discipline saves headaches. Eye and skin protection, fume hoods, and basic hygiene routines are non-negotiables. A careless transfer could result in skin rash or worse, even without clear-cut evidence of chronic harm.
Colleagues often joked that the scariest chemicals were the ones nobody bothered to fully study. The wise old supervisor always said, “Respect what you don’t know.” Stories circulate in chemistry circles about surprises—hidden toxicity, cumulative effects nobody saw coming—that only show up with long-term use. TMPO doesn’t bear skull-and-crossbones on every sheet, but the absence of clear bad news doesn’t equal good news.
What’s really missing: thorough, peer-reviewed studies on chronic exposure, environmental persistence, bioaccumulation, or breakdown products. Most specialty chemicals hover in regulatory gray space unless usage skyrockets or a headline grabs attention. It falls on occupational health teams to fill these blanks, relying on sound lab practices.
The American Conference of Governmental Industrial Hygienists (ACGIH) and similar groups set exposure limits for known toxins; for something like TMPO, the “as low as reasonably achievable” approach takes over. Without clear data, most companies err on the side of caution. Frequent training, close reading of each new product sheet, and investing in proper personal protection can make the difference between a safe lab and dangerous guesswork.
Better hazard labeling and more transparent publication of negative results would help. Regulatory agencies gain little incentive to flag obscure chemicals unless reports mount. The chemistry community grows stronger when researchers share not just breakthroughs but documentation of no-effect or oddball side effects. Investing in toxicity screening—even at small scale—gives every chemical handler a better shot at safe decision-making. Chemical makers and research leaders can push for these studies before a substance finds wide adoption.
No test tube or flask justifies risking personal health by gambling on a blank chemical safety profile. Every new, or rare, lab ingredient benefits from skepticism, vigilance, and a team’s commitment to safety.
Trimorpholinophosphine oxide isn’t the kind of chemical you leave lying around. Any scientist or technician who has ever worked with tricky chemicals knows just how important it is to pay attention to storage. This isn’t just about ticking a box on a safety checklist. One mistake can ruin months of work, cause contamination, or, worst case, send someone to the hospital. Real-world experience in research labs has shown that problems with storage catch up eventually. Leaks, accidental mixing, or simple negligence can spell disaster.
This compound holds its structure well enough under the right conditions, but exposure to moisture, heat, or random chemicals can set off reactions nobody wants to deal with. Both researchers and workers face risks when bottles sit open on a counter or wind up in a cabinet next to incompatible substances. I’ve seen labels fade and containers weaken in poorly ventilated rooms. Lab audits often reveal classic mistakes, like storing sensitive materials next to acids, or failing to double-check the temperature on a refrigerator. It’s all preventable.
Best practice means choosing a cool, dry, and well-ventilated place. A tightly sealed container cuts down the risk of moisture sneaking in or vapors escaping. I remember one incident where a poorly closed cap led to a small spill—and a big headache. Labeling every bottle clearly with both the name and the date of receipt helps staff catch problems before they develop. Chain of custody matters here, too; once I watched a colleague sort out confusion over unlabeled stock that could have thrown off an entire set of experiments.
Storing incompatible materials together spells trouble. Common sense tells you not to put flammable or reactive compounds nearby. If a lab regularly handles different categories of chemicals, separating storage sections cuts down on chance incidents. This takes discipline. Weekly checks in our lab make sure nothing gets forgotten at the back of the shelf.
Regulation stands behind all of this—OSHA and the Globally Harmonized System provide clear advice on handling and storing hazardous substances, and for good reason. Past accidents have shown how shortcuts lead to harm. Serious researchers follow Material Safety Data Sheet (MSDS) guidance on every item. A solid point: ventilation matters just as much as location. Locked, ventilated storage cabinets mitigate risks and discourage unauthorized access. Access control isn’t just about stopping theft—it’s about safety for everyone in the building.
The right procedures reflect respect for both people and property. Training staff, updating protocols, and using spill-control materials says a lot about a lab’s attitude. In practice, labs with good habits rarely see accidents. Real voices advocate for practical improvements: regular training, clear checklists, and backup supplies for emergencies. I’ve seen teams avoid catastrophes by simply double-checking before leaving for the night. Spotting signs of age on a chemical bottle or noticing granules in a liquid means someone is paying attention.
All the reminders about storage make a difference. Tight routines, proper containers, and correct placement reduce waste, save money, and most importantly, keep everyone safer.
Anyone who works in labs, particularly in synthesis or specialty projects, hears about niche compounds like Trimorpholinophosphine Oxide. That sort of name pops up in academic papers, patent filings, and industrial development. Accessing chemicals like this involves more than clicking “add to cart”—I’ve felt the frustration of searching for reliable sources for specialty reagents.
Trimorpholinophosphine Oxide isn’t something hardware stores keep on their shelves. Most buyers start the journey online. Major chemical suppliers—Alfa Aesar, Sigma-Aldrich, TCI Chemicals, and ChemShuttle—typically carry a broad stock of organophosphorus compounds. Their catalogs cover the basics, but also those rare reagents someone needs once a year. These companies prioritize material traceability, rigorous documentation, and purity testing. During a previous job in pharmaceuticals, I experienced fewer delays when ordering from established suppliers, especially when the molecule wasn’t commonly requested.
There’s a catch, though. Not every supplier fills orders for individuals or small labs—many only work with institutions. Large distributors demand company or university credentials, plus paperwork confirming the chemical’s intended use. This screening keeps sensitive materials out of the wrong hands. Buyers without institutional backing sometimes encounter dead ends or bureaucratic roadblocks. In my experience, reaching out to customer support or local representatives can sometimes yield access that a website alone doesn’t show.
It’s tempting to hunt better prices or faster deliveries from lesser-known internet shops or overseas resellers. Here’s why I always slow down. Legal controls on some chemicals vary widely between countries. Customs may detain or confiscate shipments, and receiving restricted materials can trigger serious consequences. An acquaintance once lost an entire order (and several months of research) because the supplier didn’t include the export paperwork. Standard practice is to stick with companies that post certificates of analysis, lot numbers, and proof of production origin.
Researchers trading tips on professional forums like ResearchGate or Reddit’s r/chemistry help narrow the search. Sharing supplier reviews, pricing stories, and import advice lifts the fog when navigating a purchase. Through crowdsourcing, I’ve steered clear of resellers with poor packaging standards or unreliable deliveries. Sometimes you come across recommendations for ‘off-list’ suppliers—mom-and-pop distributors filling niche demand through flexible customer service and deep connections with producers. These shops often shine with specialists working on custom synthesis or scale-up requests.
Request a quote through official channels and seek out paperwork up front. Ask for safety data sheets (SDS). These steps cut risk and ensure compliance with your country’s import regulations. Track reviews: Any reputable supplier sticks by their product, handles delivery promptly, and offers transparent return policies if quality doesn’t meet lab specs.
Greater openness in chemical sales goes a long way toward helping innovation and education. Streamlined ordering for approved researchers, fair pricing, and honest inventory listings give buyers the confidence to run safe and successful experiments. Trust and access—those are the real catalysts in science today.
| Names | |
| Preferred IUPAC name | Tris(morpholin-4-yl)phosphine oxide |
| Other names |
O=P(NMorph)3 Tris(morpholino)phosphine oxide Phosphine oxide, trimorpholino- |
| Pronunciation | /traɪˌmɔːr.fəˌliː.noʊˈfɒs.faɪn ˈɑk.saɪd/ |
| Identifiers | |
| CAS Number | 4431-76-1 |
| 3D model (JSmol) | `3Dmol('CCN1CCOCP1=O')` |
| Beilstein Reference | 4165685 |
| ChEBI | CHEBI:33288 |
| ChEMBL | CHEMBL151809 |
| ChemSpider | 120692 |
| DrugBank | DB15354 |
| ECHA InfoCard | ECHA InfoCard: 100.044.284 |
| EC Number | 21936-38-9 |
| Gmelin Reference | 82273 |
| KEGG | C20960241 |
| MeSH | D014267 |
| PubChem CID | 11346838 |
| RTECS number | TL5950000 |
| UNII | 9G7U4H16D7 |
| UN number | UN3276 |
| CompTox Dashboard (EPA) | DTXSID10885646 |
| Properties | |
| Chemical formula | C12H27N3OP |
| Molar mass | 222.26 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.179 g/cm³ |
| Solubility in water | Soluble |
| log P | -1.16 |
| Vapor pressure | Vapor pressure: 0.0081 mmHg (25°C) |
| Acidity (pKa) | 13.7 |
| Basicity (pKb) | 1.45 |
| Magnetic susceptibility (χ) | -65.0 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.510 |
| Dipole moment | 4.51 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 253.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -778.9 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and serious eye irritation |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06, GHS08 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements: P261, P280, P304+P340, P312, P405, P501 |
| NFPA 704 (fire diamond) | 1-1-1 |
| Flash point | > 174°C |
| Autoignition temperature | 220°C |
| Lethal dose or concentration | LD50 (oral, rat): 2370 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Trimorpholinophosphine Oxide: "LD50 (rat, oral) > 2000 mg/kg |
| NIOSH | NA |
| PEL (Permissible) | Not established |
| REL (Recommended) | 2 to 8°C |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Hexamethylphosphoramide Triphenylphosphine oxide Tris(dimethylamino)phosphine oxide Tris(pyrrolidino)phosphine oxide Tris(piperidino)phosphine oxide |