Looking back, Trimethylsilylimidazole (TMSI) barely got a mention outside a handful of synthetic chemists’ circles when it appeared in the mid-20th century. It started out as part of the broader chase for reagents that could speed up and simplify the process of converting organic compounds into forms that worked better with new analytical techniques emerging in the postwar boom. Researchers who spent long days wrangling with classical derivatization methods—often messy, slow, and unpredictable—saw in TMSI a wildcard: a molecule that changed how folks handled functional group protection and silylation. Moving across decades, once the value in gas chromatography became clear, manufacturers started refining the making and packaging so labs in industry and academia didn’t have to cook it up from scratch.
Grab a bottle of Trimethylsilylimidazole and you’ll see a clear, colorless liquid that comes with both promise and some caution. It works as a silylating agent and is popular among analytical chemists. The main draw? TMSI modifies small functional molecules—everything from carbohydrates to steroids—so detectors read them more easily during gas chromatography. Folks who use it will find it strong, quick, and reliable for this job. Unlike bulkier, more reactive agents that can leave too many byproducts, TMSI seems streamlined, with a knack for producing predictable results, which matters most when time and accuracy drive every step.
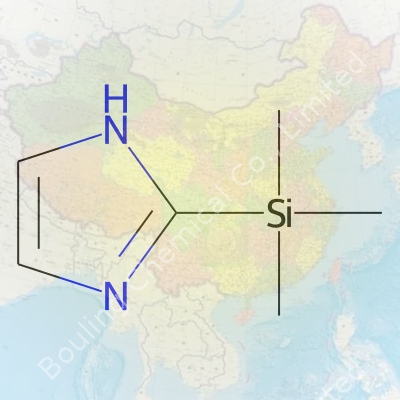
Looking closer, TMSI shows a boiling point near 123–125°C and a density right around 0.91 g/cm³ at room temperature. With its formula C6H15NSi, it belongs to the broader family of silylating agents, packing a pungent, almost sharp odor that signals its presence even before a label confirms it. Its affinity for reacting with compounds containing hydroxyl, carboxyl, or amino groups means it unseats older reagents in GC sample prep. The structure—a trimethylsilyl group tethered to imidazole—gives it a perfect balance of volatility and reactivity.
Most bottles list minimum assay values of 98% or higher. Labels describe the main hazards—flammable liquid, may cause serious eye irritation—and highlight recommended storage out of strong light, air, and moisture. Safety Data Sheets go into detail: TMSI fumes and spills ask for good ventilation, fire-resistant storage, and gloves plus goggles during transfers. Whether it comes from bulk chemical companies or niche suppliers, the consistent labeling and barcode traceability routines reflect decades of lessons, some learned the hard way, about handling safety and regulatory compliance.
The route to TMSI often runs through a reaction between chlorotrimethylsilane and imidazole in a basic medium, commonly using an organic solvent like tetrahydrofuran. Stirring, gentle heating, and solvent removal produce a liquid that’s ready for distillation and purification. Every old organic chemistry hand has seen the byproducts—hydrogen chloride catches in the back of your throat, but if everything’s dialed in right, yields stay high and side reactions low. While some labs make their own, commercial processes have scaled this up, adding robust purification steps to meet the purity needed for advanced analytical work.
TMSI’s reputation comes from its ability to transfer the trimethylsilyl group to alcohol, carboxylic acid, and amine groups, turning them into stable, volatile derivatives. This matters in real-world analysis—take a sticky sugar, treat it with TMSI, and suddenly it’s ready for volatilization and detection. Chemistry professors like showing students how TMSI bypasses tedious esterification steps. The mechanism often involves imidazole acting as both nucleophile and leaving group, which smooths out the reaction’s pace. Strength lies in its efficiency—fewer side-products and cleaner reaction profiles compared with older silyl chlorides or hexamethyldisilazane reagents.
Depending on who catalogs it, TMSI pops up under a handful of alternate names: 1-Trimethylsilylimidazole, N-Trimethylsilylimidazole, or even Trimethylsilyl-imidazole in some catalogs. Chemical suppliers use these interchangeably, though lot numbers, packaging sizes, and grades can differ widely. Some may bundle TMSI in GC-grade kits or pair it with ultradry solvents. For cross-border work, international names appear on documentation, each following strict rules laid out by regulators.
Handling TMSI draws attention from safety officers and lab veterans alike. The flammability—flash point below room temperature—means storage stays away from heat sources or open flames. Protective wear isn’t optional; long sleeves, gloves rated for organic solvents, and splash goggles must come out every time. Many companies enforce working under fume hoods, and lab checklists drill home the importance of spill kits and proper waste disposal. Anyone breathing the fumes for too long risks headaches or worse, so good practice involves monitoring lab air quality and training up on spill response. Fire marshals and chemical industry standards committees both keep close tabs, since historic mishaps have pushed new best practices into everyday routines.
Trimethylsilylimidazole’s zone of influence rests in analytical chemistry, especially gas chromatography and mass spectrometry workflows. It turns polar, sticky molecules into slick, easily detectable ones. Environmental labs lean on TMSI to analyze pesticide residues. Forensic chemists, checking toxicology samples, reach for it to make elusive compounds show up clearly. Research into natural products—plant sterols, fatty acids, even amino acids—relies on TMSI to keep sample handling efficient. It also shows up in pharmaceuticals, where characterizing intermediates gets easier once functional groups have a TMS tag. Some protein and peptide researchers use it for side-chain modification, taking advantage of its selectivity and speed.
Innovators haven’t let TMSI settle into routine status. Teams keep exploring how it can tweak DNA, proteins, or synthetic polymers for more accurate characterization or for surface modification projects. Comparative studies look at reaction rates, product profiles, and compatibility in automation platforms. Green chemistry pushes seek ways to reuse or recover spent TMSI. As automation spreads in high-throughput labs, methods for pre-packaging TMSI in safe, stable formats have emerged, reducing handling risks while keeping up with demand for rigorous, repeatable sample prep. Some ongoing work pushes for bio-based feedstocks to make the reagent, though classic petrochemical routes remain dominant for now.
Years of animal and in vitro studies confirm TMSI’s hazards. It irritates mucous membranes and can damage lungs if inhaled in heavy concentrations. Toxicology screens show acute oral and dermal toxicity, with LD50 values guiding how labs store, use, and dispose of the chemical. Regulatory bodies have written up detailed risk assessments, and today’s bottles bear warning symbols for everything from eye irritation to chronic toxicity concerns. While rare, accidental contact and spills have driven home how vital good safety culture is—both at the bench and in warehouses. Regular training, clear signage, and systematic incident reporting all contribute to keeping incidents low.
The next chapter for TMSI points to broader uses: microfluidics and portable analysis devices need silylation agents that are stable and easy to automate; some biotechnology startups weigh reformulation for DNA and RNA sequencing tools. Calls for greener, less hazardous derivatization are growing louder. Synthetic chemists push for analogues that offer similar performance but improved environmental profiles. Big pharma keeps one eye on regulatory frameworks and another on next-generation analytical platforms, expecting TMSI-standardized protocols to anchor quality control. Private and public labs team up with manufacturers to develop packaging that reduces user exposure while maintaining reagent stability. After decades as a workhorse, TMSI keeps finding new roles as demands for accuracy and safety intersect with pressures for sustainability and automation.
Step into any decent analytical laboratory, and bottles of chemicals line the shelves—each with a purpose. Trimethylsilylimidazole, or TMSI as it’s often called, sits among them. Odd name, but this stuff gets real work done. Folks who poke around with GC-MS (gas chromatography-mass spectrometry) know its value. Organic molecules don’t always play well with chromatography machines. Some won’t evaporate easily or they show up as smeared peaks, which makes interpreting data a chore. Drop TMSI into the mix, and things start to shift.
Say you want to test blood or food for sugars, fatty acids, or drug residues. Direct injection of samples would be wishful thinking; messy mixtures and sticky, polar compounds clog things up. TMSI steps in to convert these sticky groups—like acids and alcohols—into something more volatile and less reactive. The process, called silylation, replaces those hydrogen-heavy spots on a molecule with trimethylsilyl groups, turning practicality nightmares into reliable peaks on a chart. I’ve watched analysts try to get meaningful data from untreated samples. Frustration follows. After a quick TMSI treatment, peaks sharpen, ghost signals fade, and everyone’s jobs get a bit easier.
It isn’t just about making simple sugars easier to measure. TMSI has this knack for working with stubborn chemicals that resist routine analysis. Amino acids, steroids, pesticides—TMSI helps to silylate them. It stands up to rivals like BSTFA and MSTFA, but TMSI is often the go-to for transforming tricky molecules that react poorly with ordinary silanizing agents. Some friends in organic synthesis tell me it even pulls weight as a mild base for certain reactions. It does more than make life easier for the person at the computer.
No chemical is a magic solution. TMSI burns the nose, irritates skin, and isn’t gentle if splashed around. Fume hoods, gloves, and goggles turn into habits rather than afterthoughts. Mishandling costs time and health. Labs with good safety track records keep TMSI under rigid protocols, and accidents dwindle because a bit of common sense pays off. Disposal creates its own headache; careful neutralization and collection matter more than ever in a world focused on environmental footprints.
Every well-run crime lab, medical diagnostic setup, or food quality shop eventually runs into unruly samples. The cool thing about TMSI is that it gets answers from materials that would’ve sat unresolved. Reliable lab results drive important calls on health, safety, and legal trouble. In my own years working on contaminated soil and groundwater, clear data separated worry from false alarm. TMSI helped stack the odds in our favor. It won’t ever be a household item, but plenty of communities count on its chemistry to push the work forward.
Simplicity tempts anyone looking to cut corners, but new instruments and better-trained hands never replace thoughtful chemistry. Keeping up with safer, more eco-friendly reagents would help. Finding options less tough on people and the planet should stay at the top of chemists’ to-do lists. Until then, TMSI gets the job done for labs that need sharp answers from complex stuff.
Digging into the world of chemical reagents, you find some names pop up more than others during lab work. Trimethylsilylimidazole commonly joins the discussion. Its chemical formula is C6H12N2Si. To make sense of that, the molecule hooks together an imidazole ring and a trimethylsilyl group. In practice, this structure often lands a spot on the bench for its help with silylation.
It’s one thing to spot a name on a bottle and quite another to reach for it in the thick of a long experiment. Trimethylsilylimidazole steps in when folks hope to modify compounds with silyl groups. Think about workups in analytical chemistry—GC and mass spectrometry demand volatility, and polar functional groups like alcohols or acids in your sample won’t fly. A quick batch of silylation, and those compounds glide through instruments without splitting or sticking.
The demand for clean, fast derivatization never really shrinks. Over in the biological sciences, or when chasing environmental contaminants, accuracy leans not just on the machines, but on the prep work. I learned that after prepping herbicide residues in water samples. The instructions called for trimethylsilylimidazole to treat those stubborn acids. Before its addition, detecting some analytes looked nearly impossible. Afterward, signals sharpened and peaks turned clear as day.
Anyone reaching for a bottle in the stockroom knows the nerves that come with unforgiving reagents. This one carries a sharp odor and has a knack for irritation. Gloves, goggles, and fume hoods aren’t optional. Years ago, a split-second lapse meant a cough that wouldn't stop and clean-up late into the night. No one forgets that sort of lesson.
Storage turns into a bigger headache if humidity creeps in. Moisture breakdown means waste, both costly and time-consuming. Research labs already lose enough to short shelf lives and botched syntheses. Quality assurance takes more than a label on a box. Routine checkups, weight monitoring, and tight caps keep things from going south before a week is up.
Many groups struggle with waste management. Trimethylsilylimidazole doesn’t win awards for green chemistry friendliness. Runoff can’t just go down the drain. Labs push for safer disposal, but the tricky part lies in balancing reliable chemistry with safer byproducts. Some look into alternative reagents, but nothing matches its punch—at least, not for every reaction.
Safer, less toxic alternatives remain a tall order. Sometimes an extra purification step means half an afternoon lost. We need solutions that keep workflows humming along, not smothered by red tape or squeezed by extra cost. For now, respect for the reagent and strict protocol stick as the best advice. More open sharing of practical mishaps could drive safety innovations faster than a stack of dry technical reports. After all, most progress in the lab comes less from the textbook and more from the people swapping stories after a long day in the hood.
Trimethylsilylimidazole—let’s call it TMSI for short—is not just another bottle tucked away on a shelf in a laboratory. If you’ve ever watched a colorless liquid quietly evaporate and wondered what might be swirling into the air, you’ll know the subtle power some chemicals hold. TMSI lands in that territory. People use it for silylation, especially in analytical chemistry, and it makes short work of making molecules a bit more cooperative for analysis. Mistreat this liquid, and problems stack up fast.
Walking into a lab after someone’s handled TMSI the wrong way, you notice the sharp, ammonia-like scent first—a cue to double-check if the caps are tight and the containers are where they belong. Left sitting out, TMSI draws in moisture. Water-laden air starts breaking it down, releasing fumes that nobody wants to breathe and gumming up the reagent for future use. Bad storage is more than a mess; it leads to wasted chemicals, lost samples, and headaches ranging from minor to severe.
Forget about fancy containment for a minute. Best practice means clear labeling, using glass bottles with tight seals, and parking them in cool, dry spaces. TMSI’s vapor pressure climbs with warmer temperatures, so shelving this stuff next to a radiator or near sunlight shoots risk through the roof. I remember a research assistant who once left a sealed bottle near a sunny window; the container ended up bulging slightly by the afternoon and had to be disposed of as a precaution. No one wants an unplanned cleanup wrapped in safety tape.
Anyone who’s opened a reagent bottle and found a crusty ring around the rim knows what’s happened: moisture sneaked in, kicked off a reaction, and the chemical lost its edge. Humid environments ruin TMSI in a hurry. Rushing through cleanup, leaving a bottle slightly uncapped, or grabbing it with gloves damp from water causes silent, costly degradation. It pays to store TMSI with desiccants. Desiccant packs sit quietly in cabinets, pulling stray moisture out of the air. No one notices them until someone forgets to replace them and the chemistry starts going sideways.
Leaving TMSI around, open or in damaged bottles, turns carelessness into real risk. Vapors irritate the nose and eyes and will send anyone coughing for the exit if concentrations climb high enough. One strong whiff drives home the lesson that fume hoods aren’t optional. Storing TMSI under a fume hood after use, wiping down threads, and making sure containers close firmly can stop the majority of accidents before they start.
For storage over the long haul, cold storage with reliable seals stands out. I’ve seen labs stash bottles in fridges marked specifically for chemicals, not lunches. This step limits evaporation and keeps fumes at bay, even during summer. Safety data sheets don’t just tick boxes—they tell real stories written in accident statistics. Pouring over them before ever unsealing a bottle makes all the details stick: glass, cool, dry, sealed, separate from acids and bases, with easy access for those who actually use it.
No shelf or fridge keeps chemicals safe if people ignore the basics. Training newcomers isn’t just a bureaucratic box to tick. It comes from the reality that smart storage habits grow out of small, daily choices. Whenever someone sees a bottle out of place or a seal left loose, fix it. Cultures where folks speak up about storage set the groundwork for safe, productive labs. TMSI sticks around in research for good reason, but so does the need for careful, consistent storage practices, day after day.
Trimethylsilylimidazole, or TMSI, does the rounds in plenty of labs because it makes certain chemical reactions easier and faster, especially when dealing with stubborn molecules. It brings ease to a tricky process, but like any useful shortcut, it comes with its own heap of risks—sharp ones if you’re not paying attention. I remember my early years grinding through organic syntheses, eager to nail results, obsessed with yields, often overlooking the hazards to chase an end point. That fog lifts quickly with TMSI on the bench.
You will know when TMSI hits the air. The smell kicks hard, almost metallic and biting—proof it’s not meant to wander down your nose. Just because you sense it doesn’t mean you’re safe. Its vapors irritate mucous membranes—eyes, nose, throat—before you even think about getting careless skin contact. A lesson many of us learn fast: gloves aren’t optional, and not every glove blocks chemicals like this. Nitrile works better than latex, especially against strong irritants.
If you ask around in any working lab, safety glasses hang on ears for more than show. TMSI splashes, and nobody wants to risk corneal burns. Goggles, the kind that hug your face, do more than standard eyewear. Plenty of bottles have suffered cracks or sticky drips from a careless hand, and the extra seconds pulling on tight-fitting goggles beats hours of hospital paperwork and permanent eye trouble.
Open benches tempt shortcuts, but that’s where trouble brews. Fume hoods become your best bet with TMSI and any volatile reagent. I’ve watched colleagues suffer itchy throats and headaches, all from skipping this basic move. It doesn’t take much. Work inside a functioning fume hood, keep the sash low, and never reach over an open flask.
TMSI reacts with water, sometimes catching folks off guard with sudden heat or splatter. Moisture lurks in glassware, rubber septa, and even cheap gloves. Dry everything: glassware, syringes, even the spatula. I’ve seen more than one experiment stall or spiral into mess because someone underestimated a bead of condensation. It isn’t all about dramatic accidents—small releases and ruined runs cause enough regret already.
Cleanups run smoother if you plan ahead. Seal TMSI containers tightly, never leave them open, and label everything in plain language. Waste contains reactive leftovers, so don’t toss it in the regular trash or let it linger on a bench. Store the waste separately, away from acids and bases, and let the disposal folks handle the rest.
Simple changes save skin and nerves: glass pipettes over plastic, slow pours over quick dumps, and a hand on the container every time you measure. Spills happen fast, so keep a running supply of spill pads and neutralizing agents nearby—no one wants to scramble for them during an emergency. Training matters as much as gloves or goggles, so share your dumb mistakes with new lab members. Open talk keeps everyone sharp.
Working with trimethylsilylimidazole, or TMSIM, in the lab, I quickly realized that its quirks can catch you off guard. Folks love TMSIM because it’s a handy reagent for silylation—putting on trimethylsilyl (TMS) groups to turn polar, hard-to-handle compounds into something more volatile and friendlier to analysis, especially in gas chromatography. No matter how useful it is, the myth of universal compatibility with any solvent doesn’t hold water.
The moment water touches TMSIM, you’ll notice a problem. TMSIM reacts with water, breaking down and forming byproducts that interfere with silylation. Even a tiny bit of moisture in a solvent can disrupt the process. Take acetonitrile: it’s a favorite in many labs, but unless bone dry, it quickly derails your reaction. Ethanol and methanol? Those alcohols eat up silyl groups fast, meaning TMSIM just won’t work there.
My own work with aromatic hydrocarbons like toluene or even dry dichloromethane runs smoothly with TMSIM. Non-protic, dry solvents support the silylation without pulling the rug out from under the reaction. Diethyl ether or hexane, when dried well, also keep things in check, avoiding issues with unwanted hydrolysis or side reactions.
Some chemists might get away with using dry acetonitrile or pyridine. Each lab settles on what works, but the rule’s pretty simple: strong non-protic, non-nucleophilic solvents let TMSIM do its job. The moment you veer into water-loving territory, or tarnish your mix with alcohol, you set off a domino effect leading to less silylation and more frustration.
There’s a moment every researcher experiences: you come back to skimpy yields or strange peaks on your chromatogram. In my early days, I lost hours troubleshooting conversions that crashed, only to notice my solvent bottle sweating in humid air. TMSIM doesn’t forgive a lapse in solvent care. You’ll burn through reagents and samples, and sometimes the cleanup brings its own headaches.
A little diligence up front saves a lot of effort. Always test your solvent dryness. If you’re getting inconsistent results, swap out for fresh, sealed solvent and run without delay. Even better, add a drying agent before you set up. It’s tempting to skip these steps in a rush, but the fallout eats up more time and supplies than that extra carefulness ever did.
Colleagues in teaching labs often introduce extra precautions: using freshly distilled solvents and glassware dried in an oven before use. These steps might seem fussy or old-fashioned, but they rarely let TMSIM down. I still follow these habits, especially when a big batch depends on a smooth, reliable reaction.
Anyone hoping for a single fix, a solvent that always works, leaves disappointed. TMSIM’s picky nature teaches chemists to handle their tools with respect and observation. Treating solvent selection like it’s just a checkbox to tick invites problems every time. Get your solvents right, and TMSIM transforms stubborn polar molecules in a blink—cut corners, and you chase your tail for hours. That’s the wisdom experience, not just a datasheet, brings to the bench.
| Names | |
| Preferred IUPAC name | 1-trimethylsilyl-1H-imidazole |
| Other names |
1-Trimethylsilylimidazole N-Trimethylsilylimidazole TMSI N-Trimethylsilyl-imidazole |
| Pronunciation | /traɪˌmɛθɪlˌsɪliˌɪmɪˈdaɪzoʊl/ |
| Identifiers | |
| CAS Number | 18156-74-6 |
| 3D model (JSmol) | `3D model (JSmol)` string for **Trimethylsilylimidazole**: ``` CC[Si](C)n1cncc1 ``` |
| Beilstein Reference | 1201832 |
| ChEBI | CHEBI:53156 |
| ChEMBL | CHEMBL37343 |
| ChemSpider | 58842 |
| DrugBank | DB08615 |
| ECHA InfoCard | 100.023.610 |
| EC Number | 220-980-7 |
| Gmelin Reference | 7745 |
| KEGG | C09710 |
| MeSH | D017937 |
| PubChem CID | 69121 |
| RTECS number | TI5950000 |
| UNII | Q4QFK6EE8K |
| UN number | UN1993 |
| CompTox Dashboard (EPA) | DTXSID7043896 |
| Properties | |
| Chemical formula | C6H15N2Si |
| Molar mass | 142.27 g/mol |
| Appearance | Colorless to yellowish liquid |
| Odor | Characteristic |
| Density | 0.962 g/mL at 25 °C |
| Solubility in water | Decomposes |
| log P | 0.65 |
| Vapor pressure | 1 mmHg (20 °C) |
| Acidity (pKa) | pKa = 7.0 |
| Basicity (pKb) | pKb = 7.11 |
| Magnetic susceptibility (χ) | -69.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | n20/D 1.424 |
| Viscosity | 1.021 mPa·s |
| Dipole moment | 3.49 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 324.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4975 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07, GHS08, Danger, H226, H302, H312, H315, H319, H332, H335, H351 |
| Pictograms | GHS02,GHS07 |
| Signal word | Danger |
| Hazard statements | H226, H302, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P280, P301+P312, P302+P352, P305+P351+P338, P312, P330, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 1-3-1 |
| Flash point | 74 °C |
| Autoignition temperature | 250 °C |
| Explosive limits | 1.2–10.6% |
| Lethal dose or concentration | LD50 oral rat 1600 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1600 mg/kg (oral, rat) |
| NIOSH | JN8225000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 5 mg/m³ |
| Related compounds | |
| Related compounds |
Trimethylsilyl chloride Imidazole Bis(trimethylsilyl)acetamide N,O-Bis(trimethylsilyl)trifluoroacetamide Trimethylsilyl cyanide |