In chemistry, stories of molecular discovery often show how simple ideas have huge impacts. Trans-4-Phenyl-L-Proline stepped into the limelight as chemists searched for proline derivatives to help assemble complex peptides and pharmaceuticals. Early research focused on proline’s role in protein folding. Adding a phenyl ring at the 4-position was about seeing how side chains affect behavior, not about drug development, but it quickly found its place in medicinal chemistry toolkits. Labs first produced the compound in small batches for basic research, but as demand in peptide synthesis grew, chemical suppliers ramped up production and standardized manufacturing. Large companies published technical sheets about it before 2000, and soon patents appeared using Trans-4-Phenyl-L-Proline in drug synthesis. The molecule now serves as a workhorse for researchers and companies looking to design new drugs with improved biological properties.
Trans-4-Phenyl-L-Proline is an amino acid derivative that’s recognized by a unique twist in its structure: the four-membered proline ring gets a phenyl group stuck on at carbon four, defining the "trans" orientation. Off-white powder, sometimes crystalline, it dissolves easily in water and polar solvents. More rigid than plain proline, it resists flattening under stress, holding shapes that lock in special combinations during peptide formation. Chemical suppliers sell it in both small research vials and bulk drums for industrial use. The compound is available as a stable, shelf-friendly solid and ships under room temperature conditions. Labels usually list CAS number 3077-48-1, purity of 98% or higher, and sometimes even optical rotation values, since chirality defines the whole point of this molecule.
You can spot the physical traits of Trans-4-Phenyl-L-Proline pretty easily. The molecular weight comes in at 203.25 g/mol, and the melting range hovers around 203-206°C. Its chirality makes it optically active — rotate polarized light through a dissolved sample and you can see those degrees shift based on the L-isomeric purity. The rigid ring adopts a puckered conformation, adding to the stability of peptides built with this amino acid. Basic solubility works best in water and methanol, with some compatibility with DMSO for demanding synthetic reactions. Chemically, the compound resists hydrolysis under normal lab conditions but reacts neatly with activators during peptide coupling. Since phenyl groups are hydrophobic, this molecule slightly boosts nonpolar character when part of a peptide chain.
Chemists working with Trans-4-Phenyl-L-Proline pay attention to manufacturer data sheets, focusing on several technical details. Purity usually exceeds 98%, often determined by HPLC or NMR. Moisture content is kept under 0.5% by weight, and labels mention the specific optical rotation, an important factor for chiral purity. The major supplier codes and product catalog numbers keep reappearing in the literature, making it easy to trace and compare batches. Labels include recommended storage conditions – typically room temperature in a tightly sealed container, away from sources of moisture. The molecular formula C11H13NO2 and InChI key also pop up for database searches. Compliance with international standards like ISO or REACH forms a point of trust for buyers handling sensitive medical research.
Most commercial production runs start from L-proline itself, using a stepwise synthesis to tack on the needed phenyl group at the fourth position. One common route uses palladium-catalyzed coupling reactions, where the proline ring gets functionalized and the phenyl ring attaches via an arylation. Protecting groups shield the amino and carboxylic acid sites, stopping unwanted reactions at those spots. Deprotection steps at the end bring the molecule to its final form. Reaction conditions must be just right — temperature, solvent, catalyst loading all get carefully tuned to prevent racemization and maximize yield. Labs usually perform purification by recrystallization, then check purity on HPLC before distribution. Larger production lines might favor continuous or flow-based synthesis for better control and consistency across big orders.
Trans-4-Phenyl-L-Proline heads straight into peptide coupling reactions, often reacting with carboxylic acid-activating agents like DCC (dicyclohexylcarbodiimide) or EDC (ethyl(dimethylaminopropyl) carbodiimide). The rigid backbone and bulky side chain steer peptide folding and introduce new turns or breaks in the finished polymer. On the bench, you can run standard protection/deprotection schemes from peptide chemistry on both the amine and carboxyl groups. That means Fmoc or Boc strategies fit, so it’s compatible with automated peptide synthesizers. Reduced forms of the amino acid (making the phenyl group a cyclohexyl, for example) and additional substitutions let chemists create libraries of derivatives. Its phenyl ring invites aromatic substitutions or cross-coupling for further molecular modifications, expanding the playground for drug design.
Researchers, catalogues, and suppliers tag this substance with a slew of names: (2S,4S)-4-Phenylpyrrolidine-2-carboxylic acid, Trans-4-Phenyl-L-proline, and the short code 4-Ph-L-Pro. Sometimes you’ll see the short-hand "trans-PP," and the compound’s presence in pharmaceutical intermediates leads to further alphanumeric cataloging, especially in inventory management. Brands in the chemical supply world give it their own stock numbers, and some academic papers use abbreviations based on the three-letter amino acid code system — although "Phe-Pro" might mislead, so accuracy means checking the full IUPAC naming on chemical registries.
Handling Trans-4-Phenyl-L-Proline in the lab never gets taken lightly. Users check the SDS (Safety Data Sheet) before scooping out even a milligram, since good lab practice demands clear information about risks. It’s classified as relatively safe among amino acid derivatives, though like most powders, it poses an inhalation risk if not handled with proper ventilation or a mask. Skin or eye contact may cause mild irritation, and gloves remain standard to avoid repeated exposure. Material safety protocols require storing away from acids and bases that could degrade the aromatic or proline rings. Disposing of unused material goes by local chemical waste guidelines, and bulk handlers use fume hoods and eye protection as a matter of routine. For pharmaceutical use, GMP (Good Manufacturing Practice) certification comes into play, making sure every step from raw material to final batch meets health and safety laws.
In peptide science, Trans-4-Phenyl-L-Proline serves as a specialty tool for building in structure and hydrophobicity where regular proline falls short. Researchers use it to modify sequences in synthetic peptides, trying to improve drug properties like metabolic stability or specific receptor binding. The increased ring rigidity can slow down degradation by proteases — helpful for peptides aiming for a longer lifespan in the human body. Drug hunters testing new small molecules sometimes plug Trans-4-Phenyl-L-Proline into lead structure optimization, searching for that perfect balance between absorption and selectivity.
Academic labs also pick this compound for biomimetic chemistry, trying to copy or tweak natural proteins for new materials or biocatalysts. In recent years, it cropped up in the chemical biology field, where the altered backbone gets used to force peptides into unusual folds, helping map out protein interactions with new precision.
Active research keeps finding new roles for Trans-4-Phenyl-L-Proline in both the therapeutic and biotechnological worlds. Analytical studies measure how its presence warps peptide backbone geometry, offering a route to more resistant or targeted biopharmaceuticals. Some teams look at immune-active peptides, injecting this amino acid to see how folding changes antigen recognition. Other groups engineer enzymes using modified prolines like the trans-4-phenyl variant, aiming to tweak catalytic power for green chemistry purposes. On the technical front, improvements in asymmetric synthesis of this compound lower cost and boost access for labs without industrial-sized budgets. Newer work also considers “green chemistry” production, testing catalysts and solvents that avoid hazardous byproducts and cut down on waste.
So far, toxicity studies suggest Trans-4-Phenyl-L-Proline remains safer than many of the highly reactive amino acid derivatives crowding synthetic chemistry. Oral and dermal exposure in animals points to low toxicity, but data on chronic or high-dose exposure still falls short of what’s needed for regulatory comfort. Studies check for mutagenicity and reproductive toxicity to keep pharmaceutical development on the right side of safety standards. Every batch used in regulated drug production goes through purity and contaminant screening, keeping unexpected side products out of the supply chain. As more of this compound moves toward clinical use, both company and independent research teams will need to dig deeper on long-term impacts and metabolic fate in humans, especially if it enters the bloodstream as part of new drugs.
Trans-4-Phenyl-L-Proline will see heavier use as peptide therapeutics carve out a bigger space in medicine. Drug designers hunt for building blocks offering just enough steric bulk to tilt activity without breaking compatibility with established synthesis methods. As companies push into personalized and precision medicine, demand for boutique amino acids like this will increase. Improvements in catalytic arylation and biocatalyst-driven synthesis promise lower costs, cleaner production, and faster path to market. Researchers already tap into the molecule’s backbone-rigidifying power for molecular probes, enzyme inhibitors, and even next-generation biomaterials. Ongoing collaboration between academic chemists and industrial manufacturers can speed up safety testing, standardization, and access — cornerstones for scaling lab curiosity into real-world solutions.
Trans-4-Phenyl-L-Proline may sound like something you’d only find scribbled on an organic chemist’s blackboard. In real world laboratories, it serves as a key material in the development of many modern pharmaceuticals. Drug makers often look to amino acid derivatives to fine-tune the performance of medications. Scientists turn to this one because its unique ring shape and bulky phenyl group allow for changes in how molecules fold, interact, and function.
This compound stands out in peptide research. Since it’s based on proline, an amino acid that tends to break or bend protein chains, drug researchers can use it to mimic or disrupt protein shapes. The “phenyl” part—essentially a benzene ring—adds size and offers handles for chemical reactions. Researchers use trans-4-phenyl-L-proline for crafting enzyme inhibitors, which can block out-of-control proteins in diseases like cancer or viral infections. Preclinical studies have shown that carefully chosen modifications at the proline site often lead to drugs with increased stability and improved targeting inside the body.
Anyone who’s worked on a failing medicinal chemistry project knows that small tweaks in molecular shape can mean the difference between a useless powder and a breakthrough medicine. Trans-4-phenyl-L-proline gives scientists fresh tools to address common frustrations: weak binding, poor oral absorption, and rapid drug breakdown. In my experience, teams return to this proline variant when the original designs either fall apart in harsh stomach acids or don’t latch tightly enough onto biological targets. By slipping a phenyl group onto proline, researchers get a stiffer, more hydrophobic (water-hating) spine. This rigidity and bulkiness lets the molecule resist being chopped up by enzymes, which can stretch out a drug’s lifespan in the body.
Skeptics say these chemical tweaks sometimes make compounds harder to synthesize on a larger scale, but modern manufacturing techniques keep improving. In biopharmaceutical startups, I’ve watched collaborations between chemists and engineers drive the cost of producing special amino acids down every year, which opens new doors for patient therapies.
Peptide drugs built using trans-4-phenyl-L-proline have already left the research stage. They’re making headway in the treatment of viral diseases, autoimmune conditions, and certain cancers. Drug hunters see its value in creating molecules that not only block disease-causing proteins but also avoid triggering harmful side effects. For folks outside the science bubble, these advances show how precise chemistry tools can nudge medicine closer to a world where treatments are more effective and better tolerated.
To encourage broader access, universities and companies share protocols online and at conferences, inviting more researchers into the field. Teaching programs now cover amino acid derivatives more deeply, equipping students with modern skills to tackle tomorrow’s health problems. As demand rises, efforts focus on finding greener, less wasteful ways to produce trans-4-phenyl-L-proline at commercial scale.
For many people in science, the story of trans-4-phenyl-L-proline isn’t just about one strange-sounding molecule. It reflects the way creative, incremental changes at the smallest scale shape the future of treatment. Each new result—whether a minor improvement or a leap forward—translates, bit by bit, into more options for patients facing serious illness. That’s the kind of progress that matters in the long run.
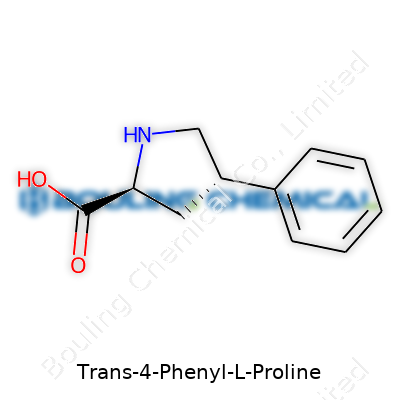
Trans-4-Phenyl-L-Proline stands out among the vast catalog of amino acids, both for its role in modern drug development and for that distinct twist in its structure. This molecule belongs to the proline family, but adds a phenyl ring sitting at the “4” position, and the “trans” designation points to the specific arrangement of its pieces in space.
Picture a five-membered ring—called a pyrrolidine. That’s the backbone for proline and its relatives. In a plain L-proline, the ring connects an amino group (-NH2) and a carboxylic acid group (-COOH), closing with three more carbon atoms. In this version, a phenyl group—a simple, six-carbon aromatic ring—attaches to the fourth carbon in that ring. In the “trans” isomer, the carboxyl group and this phenyl group point in opposite directions, like looking at the ring from above and seeing two flags sticking out on each side.
This arrangement gives the compound its unique properties. The “L” prefix says which way the amino group sits—matching the layout for most life-essential amino acids. Each part of this molecule plays a role, not only shaping how it slots into bigger biological puzzles but also how it shows up in medicine and research.
I’ve seen researchers take interest in trans-4-Phenyl-L-Proline for one clear reason: changing just a piece on proline leads to dramatic differences in the broader biological picture. That phenyl ring doesn’t just sit around for looks. It boosts the molecule’s rigidity, shrinking the number of shapes it wants to take. This matters for designing drugs. Think of protein engineering, peptide drug stability, and pharmaceutical synthesis—each benefits from molecules that lock in place, resist breakdown, and favor predictable binding.
The chemical formula for trans-4-Phenyl-L-Proline reads as C11H13NO2. Chemists can sketch its structure easily with a line drawing, or a condensed form like:
Here, “Ph” stands for that six-carbon benzene ring. The point of all these atoms is not just academic curiosity. Add a phenyl group in the trans configuration, and you change interactions with enzymes and target proteins—some of these bioactive peptides would break down within minutes without this kind of reinforcement, but with it, they hold up for hours, sometimes longer. That’s a real difference in the lab and in treatment rooms.
Skepticism over chemical tweaks sometimes gets loud in biotech. I get it: synthetic molecules often sound fancy but prove brittle in real-world use. Yet here, the evidence runs the other way. In one 2020 review, incorporation of trans-4-Phenyl-L-Proline into lead compounds made them less prone to being chewed up by peptidases. The added phenyl group doesn’t just make molecules stiffer. It offers, in certain therapeutic peptides, better oral availability and longer half-life. That’s rare in this class of drug design.
Production methods keep evolving, too. Several labs favor asymmetric synthesis routes—routes that respect the molecule’s handedness and configuration. These aren’t just academic exercises. They affect yield, cost, and environmental footprint in the pharmaceutical supply chain. With more green chemistry entering the field, the telling question is which methods trim waste and lower hazards. Such innovation feeds both safer products and more reliable mass manufacture.
Why pin so much hope on the chemical backbone of a single amino acid? Because just one substitution, done right, sends long-lasting ripples through drug discovery, protein engineering, and disease treatment. Getting structure and configuration right in molecules like trans-4-Phenyl-L-Proline changes what we can do in human health, one ring and one phenyl group at a time.
Trans-4-Phenyl-L-Proline sounds technical, but its use stretches across labs and industries. In my experience, whether something shows up in a high school chemistry set or a pharmaceutical project, how pure it is matters most. For research and production, purity shapes results and safety. Even at the basic everyday level, imagine using a tainted ingredient in a recipe—only here, errors don’t just mess up dinner, they risk expensive setbacks or failed experiments.
Over the years, when sourcing amino acid derivatives like trans-4-Phenyl-L-Proline, I’ve seen suppliers list a spread of purity grades. Lab-grade material usually sits lower—sometimes at around 97%—and works for basic chemistry jobs where minor side products won’t ruin the outcome. For pharmaceutical processes, nobody gambles; purity above 99% gets called out by the supplier, often advertised as “high-purity” or “premium-grade.” Upping the purity means extra processing, but it helps keep batches consistent and results trustworthy.
Some suppliers break it down further into analytical, reagent, technical, or research grades. Honestly, those labels don’t always line up from one company to another, and paperwork matters just as much as the number on the bottle. Certificate of Analysis gives the real details. For sensitive projects, that kind of document means you can double-check for trace metals, unwanted isomers, and water content—a lifesaver if a project demands specific conditions.
Paying up for the purest option isn’t just a matter of snobbery; in fields where contaminants spark side reactions, every decimal point counts. I remember a friend’s synthetic biology project derailed because the base compound carried trace solvents, invisible to the naked eye but deadly for cell cultures. Clean-up meant burning time and budgets. Beyond just the purchase price, choosing the wrong grade adds on the hidden costs of faulty data or whole batches tossed in the trash.
Researchers face pressure to churn out results, and labs get tempted to go with what’s on hand. But tiny differences sometimes turn simple steps into troubleshooting nightmares. I’ve learned to check data sheets and request samples. Anyone dealing with regulated products—medicine, supplements, food testing—should take that extra minute to double-check origin and batch history.
Sourcing the right product always starts with your intended application. Pharmaceutical and biotech projects thrive on transparency, so always ask for certifications, batch analysis, and impurity profiles before signing off. Don’t settle for vague promises. Talk to suppliers with direct questions: what exactly is the purity percentage, what detection was used, and what impurities remain? Insisting on this detail means long-term savings, especially when scale goes up or regulations step in.
Everyone wants things fast, cheap, and pure—but in practice, something gives. If the goal involves research at the highest level, order the premium stuff and build the extra cost into the grant or business model. For teaching or low-stakes projects, a lower grade will probably do. The trick comes in knowing which corner can be cut and which risks aren’t worth it.
Years working with chemical suppliers taught me that long-term partnerships build trust. Transparent paperwork, consistent quality, and real-time answers help weed out unreliable sources. Reputable vendors earn loyalty because nobody wants to gamble with hidden contamination. Mistakes in purity haunt results long after the shipment arrives, so the extra work upfront delivers peace of mind.
Most folks in chemistry labs learn pretty fast that mishandling chemicals brings trouble. Too much heat here, moisture there, and expensive compounds degrade before their time. Trans-4-Phenyl-L-Proline isn’t some off-the-shelf salt; it comes with its quirks. Years back, I lost nearly half a bottle in a single week after leaving it by a sunny bench. That mistake stuck with me. So, when people talk about storing such compounds, it’s less about rules and more about not wasting hard-earned resources—or worse, risking the science itself.
If you care about purity, temperature control should land high on the priority list. This particular compound prefers cooler environments. Fluctuations above room temperature increase the chances for oxidation or unwanted breakdown. At many research institutes, storage at 2-8°C, in properly maintained refrigerators, keeps degradation to a minimum. Tossing it on a regular shelf with open exposure saves seconds today, yet eviscerates budgets and projects tomorrow.
Trans-4-Phenyl-L-Proline pulls in atmospheric moisture, which doesn’t play nice with its structure. Leave the cap loose or neglect to reseal, and you risk forming clumps or seeing dissolved material later. I always recommend investing in desiccator cabinets, especially in humid climates. Silica gel packets work well and don’t cost much. Colleagues who skip desiccation usually face ruined product and awkward funding conversations. Proper drying agents keep the compound free-flowing and effective.
Bright lab lights and natural sunlight speed up breakdown. We learned to use amber vials years ago after seeing color and solubility changes under fluorescent lighting. Shielding every bottle—no matter how small—stretches the lifespan. Wrap the container with aluminum foil if amber vials aren’t available. Stored samples survive months this way without throwing off experiment results.
Open containers in busy labs threaten everything. Dust, spills, and errant powders from the next bench over sneak in without warning. I always emphasize single-use spatulas and labeling everything. Even a few grains of unknown material can throw synthesis efforts out of alignment. Clear, strict habits protect years of method development.
It’s tempting to hang on to old stock, especially for rare or expensive chemicals. With Trans-4-Phenyl-L-Proline, relying on outdated samples brings headaches. Mark purchase and opening dates on every vial. Regularly checking for clumping, discoloration, or weird odors helps catch issues early. If in doubt, testing a small amount with TLC or NMR before scaling up never hurts.
Improving storage isn’t just a check-box exercise for audits. From my time organizing underfunded teaching labs, careful labeling, strict temperature policies, and moisture avoidance led to fewer last-minute scrambles and safer environments for students. The best labs build routines so everyone knows how to keep chemicals like Trans-4-Phenyl-L-Proline stable. What’s stored well supports innovation, cuts down waste, and keeps research moving in the right direction.
Trans-4-Phenyl-L-Proline, a chiral building block used in pharmaceuticals and peptide science, isn’t just another lab shelf item. People always ask about risk—and with good reason. Accidental contact can bring headaches, nausea, or skin irritation. Inhaling dust or vapors triggers even bigger problems. I’ve worked in chemical labs where even seasoned staff cut corners, thinking “it’s just a small amount.” One slip changes your relationship with safety for good.
Nothing beats experience, but proper gear comes close. Nitrile gloves stop the compound from seeping into pores. Labs sometimes use latex, but certain chemicals eat through rubber—nitrile resists that. Safety glasses guard against splashes. Some dismiss the need for a lab coat or apron, but spills stain clothes and skin, and the chemical smell sticks around longer than any lesson learned. A properly fitted mask stops dust from entering your lungs. The best teams use well-fitted respirators—not surgical masks—when working with powders.
Ventilation isn’t optional. I remember my first year handling similar amino acid derivatives, a fume hood went faulty and the odor lingered. After that, the maintenance crew checked hoods weekly. Always use a certified fume hood, not just open windows. If the extraction is noisy, that’s fine—it means it’s working. Cross-contamination matters, too. Never eat or drink at the workbench, no matter how tempting.
Chemicals kept in unmarked flasks cause confusion. Permanent labels with the full name, the date, and hazard symbols take a minute to write but prevent mix-ups. Trans-4-Phenyl-L-Proline prefers a cool, dry shelf, away from sunlight. Direct light destroys purity. Keep it away from acids, bases, and oxidants. Never trust a crowded shelf; a single drop from a height invites a big cleanup. Store amounts needed for the week—never more.
Accidents happen even to the best. Spills demand absorbent pads or vermiculite—avoid sweeping powders into the open air. Once, a friend panicked and reached for a paper napkin; the dust just spread. Dampen then wipe to keep the particles down. Collected waste goes in clearly marked hazardous waste bins. Pour nothing down the regular drain. Partnering with a trusted chemical waste company makes life easier for everyone in the lab.
No instruction book covers every scenario. Regular training updates keep everybody sharp. Share safety bulletins. Veteran staff need refreshers as much as newcomers do. Encourage questions. Avoid private shortcuts. Every safe shift builds trust in the group—and the whole lab benefits.
Laboratory work runs on teamwork and trust. People watch out for one another. Newcomers copy what more senior scientists model. Talk about the “why” behind every rule. Mistakes happen, but the right habits help everyone go home healthy. That, in the end, is worth far more than any shortcut.
| Names | |
| Preferred IUPAC name | (2S,4R)-4-phenylpyrrolidine-2-carboxylic acid |
| Other names |
4-Phenyl-L-trans-proline L-trans-4-Phenylproline L-4-Phenylproline (trans) L-α-Amino-4-phenylpyrrolidine-1-carboxylic acid L-trans-4-Phenylpyrrolidine-2-carboxylic acid |
| Pronunciation | /træns fɔːr ˈfiː.nɪl ɛl proʊˈliːn/ |
| Identifiers | |
| CAS Number | 771-00-0 |
| Beilstein Reference | 3666393 |
| ChEBI | CHEBI:73169 |
| ChEMBL | CHEMBL137058 |
| ChemSpider | 223958 |
| DrugBank | DB08204 |
| ECHA InfoCard | 100.202.343 |
| EC Number | EC Number: 693-031-3 |
| Gmelin Reference | 808957 |
| KEGG | C02965 |
| MeSH | D065017 |
| PubChem CID | 123145 |
| RTECS number | SY7875800 |
| UNII | DB6L41IUB4 |
| UN number | UN3276 |
| CompTox Dashboard (EPA) | DTXSID5032959 |
| Properties | |
| Chemical formula | C11H13NO2 |
| Molar mass | 203.25 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.2 g/cm3 |
| Solubility in water | Slightly soluble in water |
| log P | 1.2 |
| Acidity (pKa) | 7.91 |
| Basicity (pKb) | 4.01 |
| Refractive index (nD) | 1.596 |
| Viscosity | Viscous oil |
| Dipole moment | 5.7427 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 395.9 J/mol·K |
| Std enthalpy of formation (ΔfH⦵298) | -83.6 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| Main hazards | Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS07; GHS05; Warning; H315; H319; H335; P261; P305+P351+P338 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | No Hazard Statements. |
| Precautionary statements | Precautionary statements: P261, P305+P351+P338 |
| NFPA 704 (fire diamond) | 1/1/0 |
| Flash point | > 181.1 °C |
| LD50 (median dose) | LD50: >2000 mg/kg (oral, rat) |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mM in DMSO |
| Related compounds | |
| Related compounds |
Cis-4-Phenyl-L-Proline L-Proline Trans-4-Hydroxy-L-Proline Cis-4-Hydroxy-L-Proline Trans-3-Phenyl-L-Proline |