Chemists first took an interest in thiophene derivatives over a century ago while digging into coal tar and natural products. The family grew quickly in the mid-1900s, with patents and academic works full of new functional group twists and side chains. Plugging a bromoacetyl group onto thiophene set off a wealth of reactivity, which pushed synthesis labs to explore both simple and complex routes. The path toward 2-(2-Bromoacetyl)thiophene came from a push to access new building blocks for pharmaceuticals and materials—needed by the pharmaceutical surge and the rise of specialized electronics. Over decades, researchers tuned methods to avoid harsh reagents and boost yields, creating a richer menu of choices for anyone looking to expand their synthetic landscape.
2-(2-Bromoacetyl)thiophene looks like an off-white to pale yellow powder, sometimes crystalline under the right conditions. This compound isn’t flashy in appearance, but the special blend of the bromine and thiophene ring gives both researchers and industry a smart tool for downstream modifications. Its shelf life tracks closely with proper sealing and storage; left out, it tends to draw in moisture and lose that signature sharpness in reactivity. In my own experience, this compound remains a regular candidate for synthetic planning, answering the call when one wants to reach further than standard aromatic bromides or simple acyl derivatives allow.
Solid at room temperature, with a melting point reported in the region of 65-70 °C, 2-(2-Bromoacetyl)thiophene dissolves reasonably well in polar organic solvents such as acetone and, to some extent, in ether or chloroform. Its structure draws attention, as the bromoacetyl group adds heft to the electron density of the thiophene ring, nudging, even dictating, the types of reactions you'd expect. Low volatility means you can handle it without worrying much about airborne loss, though one catch: open exposure does sometimes release an irritating odor, a warning to anyone hurrying through prep without gloves or ventilation. These characteristics steer both technique and mindset in the lab; you walk a line between respect for its hazards and excitement for its reactivity.
Supply houses tend to ship this compound at upwards of 97% purity, labeling it by both CAS number (23815-93-4) and molecular formula (C6H5BrOS). The typical bottle will carry a hazard class warning for skin and respiratory irritation—rightly so, considering the bromoacetyl group’s reputation. Bottle labels and data sheets spell out molecular weight (205.07 g/mol) and offer clear pictograms for safe handling recommendations: avoid contact, store in cool dry spots, and use only in properly hooded spaces. Manufacturers drill in the need to track batch numbers, which grows in importance for patent filings and traceability in regulated research or preclinical settings.
In the lab, I've seen this synthetic route begin with thiophene itself, followed by Friedel-Crafts acylation with bromoacetyl chloride under classic aluminum chloride catalysis. The reaction generates some heat, demanding slow addition and heavy stirring to throttle any runaway effect. Once the bromoacetyl group attaches, the intermediate must be carefully quenched and washed. Sometimes side products sneak in, so column chromatography or recrystallization takes the crude to a workable, reliable product. More modern protocols chase greener chemistry, experimenting with ionic liquids or microwave activation to dodge the mess of heavy metal residues and tedious purification. Everyone chasing new routes asks the same core question: how efficiently can you convert raw material to target without building up contaminant headaches for disposal or downstream reactions?
Transforming 2-(2-Bromoacetyl)thiophene takes advantage of both the active bromo group and the electron-rich ring. One popular play is nucleophilic substitution; the bromine swaps out under attack from amines or thiols, spinning off a host of new ligands and heterocycles. Cross-coupling steps using palladium catalysts crack open the door toward extended conjugated systems—especially meaningful for those in materials science aiming for better conductivity or charge transport. Experienced chemists keep an eye out for side reactions like elimination or undesirable overreactions, which cluster around the reactive carbonyl. Modifications benefit medicinal chemistry groups aiming to tweak biological activity or boost solubility through minor appendage switches.
Before ordering or discussing this compound, chemists bump into a stack of product names: 2-(Bromoacetyl)thiophene, 2-Thienyl bromoacetyl, bromoacetylthiophene. CAS search quickly straightens out confusion, but documentation from earlier decades occasionally flips the numbering, so careful confirmation avoids costly misorders. Some vendors shorten the name to BAT or rely on SKUs buried in supplier catalogs. Professionals and students both quickly learn to cross-reference by structure when lab stockrooms or colleagues offer up a bottle with handwritten shorthand.
Handling safety steps up with 2-(2-Bromoacetyl)thiophene, since both the bromo and carbonyl functional groups suggest strong electrophilic activity. Skin contact stings, and even modest fume exposure can irritate the eyes and throat. Lab work always starts with gloves and goggles, plus a fume hood when transferring or weighing. Disposal channels classify this material under halogenated organics, locking in the need for hazardous waste handling, not down-the-drain rinsing. Safety data sheets also set out evacuation and first aid instructions. In my own lab years, strict adherence to labeling and routine checks ensures that accidental spills don’t snowball into full-scale incidents.
This compound pops up most in pharmaceutical intermediate synthesis, offering a versatile gateway toward beta-keto derivatives, active pharmaceutical agents, and thienopyridine compounds. Medicinal chemists favor it for its fast, reliable integration into ring systems and side chains that influence drug potency or selectivity. On the material science front, incorporating thiophene units supports the design and construction of polymers—a backbone of OLED screens and organic solar panels. Its reactivity doesn’t set natural boundaries, so a clever synthetic chemist can map it into an array of applications, from dyes and pigments to agricultural chemicals.
R&D teams always look for ways to tighten up processes and lower hazards. Recent journal reports delve into catalyst-swapping, solvent-free conditions, and shorter one-pot methods for preparing 2-(2-Bromoacetyl)thiophene and its cousins. Structure-activity studies, especially in medicinal chemistry, use it to probe enzyme inhibition, anticancer potential, and new antibiotic frameworks. Researchers seeking better functional group compatibility push the envelope on reaction conditions, steadily inching away from legacy methods that relied on harsh reagents or hard-to-find starting materials. My discussions with colleagues reveal a consensus: broader, safer, and more selective syntheses will cement its role in exploratory science.
Toxicology studies reveal that the bromoacetyl group doesn’t just target lab reactions—it signals a risk of mutagenicity and acute toxicity. Animal studies flag cytotoxic effects at moderate concentrations, while skin absorption can produce systemic toxicity. Regulatory bodies such as the ECHA and EPA have required hazard labeling and control measures, including strict limits for workplace exposure. Chronic impacts remain less mapped, but chemists don’t take risks lightly, favoring routine monitoring and fastidious personal protection. Safety research keeps the door open for safer substitutes or more benign derivatives, but as things stand, 2-(2-Bromoacetyl)thiophene demands respect from every handler, beginner or veteran.
Looking ahead, the versatility of this compound signals growing value for both chemical innovators and manufacturing outfits. Digital devices, flexible electronics, and sustainable pharmaceuticals all pull from thiophene chemistry, meaning demand for reliable intermediates will likely keep pace. The biggest shift may come from process intensification—using flow chemistry or biocatalysis to lower energy use and waste. Informed by better toxicity profiles and regulatory scrutiny, future work may soon favor new analogs, offering similar reactivity but with fewer environmental headaches. The compound’s twin roles—as building block and as gateway to complexity—set a foundation for inventive science. Trends in artificial intelligence-driven synthesis and high-throughput screening will only quicken this expansion, offering new territory for both discovery and production.
In chemistry, purity isn’t just a bragging right—it shapes everything from lab results to the safety of workers handling a compound. Companies selling 2-(2-Bromoacetyl)thiophene often list purity at 97% or even higher, but it's worth digging deeper. Few professional buyers just take that number at face value. They want certificates of analysis, batch data, and sometimes run their own NMR or HPLC checks. That’s because, if you’ve ever worked in a research lab, you know contaminants mess up syntheses, foul up results, and make reproducibility a nightmare.
A synthesis route using 2-(2-Bromoacetyl)thiophene—like building thienyl-based pharmaceuticals or conducting a simple alkylation—depends heavily on the expected purity. Less-than-clean batches bring along unknown byproducts. Imagine discovering an unexpected tar or a persistent ghost peak on your chromatogram, only to backtrack the problem to a “slightly off” precursor. That costs time, money, and sometimes your nerves. For those scaling up reactions, this translates into wasted solvents, more hours debugging protocols, and products that need expensive purification steps afterward.
Most suppliers advertise 96-98% purity for 2-(2-Bromoacetyl)thiophene. From firsthand experience, those last two to four percent matter. Impurities come in many forms: unreacted starting material, side-products, solvent residues, even water. Each batch tells a slightly different story. Sometimes you find off-color crystals where white should be. Smells can be off too—the sharpness just isn’t quite right. Purity claims without backup data don’t mean much; the real work involves checking MS or NMR spectra, even a simple TLC plate, to confirm things look right before any meaningful experiment begins.
A lot depends on the synthesis route and how carefully suppliers handle the work-up and purification. Rushed extractions or poor control during distillation let through more than just the product. In some smaller shops, shortcuts show up as higher levels of related impurities. Those buying compound online notice price swings: the cheapest usually cut corners somewhere. Anyone looking to get consistent results needs to pay more for higher purity. There’s a direct correlation—higher cost often means better control from synth to storage.
The only sure path forward involves never relying on a label alone. Always request a certificate, look for real analytical data. Running a quick TLC, GC, or NMR takes minutes but can save a whole project. If trouble shows up partway through a protocol, trace the problem backward through all key chemicals and reagents. Don't hesitate to reach out to suppliers with questions—they offer batch-specific data for serious buyers. It's also worth setting aside small reference samples from each batch for side-by-side checks when problems creep in. Little habits like these separate headache-filled runs from the smooth ones.
Chemicals like 2-(2-Bromoacetyl)Thiophene don’t exactly demand the attention of the general public, but in research labs and production sites, overlooking the basics can land you with stubborn headaches. I’ve watched brilliant projects fizzle out just because someone assumed “shelf stable” meant “throw it in a cupboard next to the coffee”. That’s no way to handle compounds that can turn tricky if left on their own.
2-(2-Bromoacetyl)Thiophene isn’t on the list of wild explosives or highly volatile organics, but it still carries certain risks. There’s a bromine group and a carbonyl in there—the sort of functional groups that don’t always play nicely with moisture, sunlight, and shifting temperatures. Anyone who has worked with halogenated or acetyl-containing molecules knows the routine: swap carelessness for certainty, or lose both time and money retracing your own footsteps.
Leaving this compound out at room temperature might seem harmless. Yet labs often run warm, especially with equipment cranking away. Heat can fuel degradation, knocking purity down a peg while introducing new byproducts. From years of seeing samples baking on forgotten benchtops, I suggest building a cold storage habit—4°C isn’t overkill. This slow-down on chemical changes keeps your supplies trustworthy.
Moisture sneaks past lids and turns stable chemicals into unpredictable messes. Given the molecular makeup of 2-(2-Bromoacetyl)Thiophene, even a hint of water sets off questions about side reactions. Anhydrous conditions—using a desiccator or moisture barrier (like silica gel packs)—give you a solid line of defense. Make it a rule: tight, sealed containers, always.
Light, especially UV, brings its own trouble. I’ve seen UV creep through thin plastic or clear glass, enough to nudge a sensitive reagent off course. Opaque bottles or amber glass bottles, tucked away from windows or bright lights, make a difference. You can tell colleagues to be watchful, but real discipline means swapping out transparent storage for proper shielding.
Clean, straightforward labeling rarely pays off immediately, but it’s your friend down the line. I once spent half a morning sorting through identical vials, and every faded label cost us energy and good mood. Dates matter—log them. Don’t skip catalog numbers, storage recommendations, and your initials. If someone else comes looking, they will thank you for sparing them a guessing game.
Handling 2-(2-Bromoacetyl)Thiophene with gloves and a lab coat isn’t simply hygiene—it’s habit-building that shields you from careless splashes and trace skin exposure. Routine cleanup after each use and staying alert to spilled powder makes a world of difference. Watching a peer slouch through safety routines once led to a project freeze for weeks; small details like proper storage save time and keep teams on track.
Science is already full of unknowns. Turning storage into an afterthought brings just more variables and setbacks. Building good storage habits and creating enzyme-proof, moisture-free, cool spaces isn’t bureaucracy. It’s investment in reliable results, project momentum, and a bit more peace of mind for folks who’d rather spend their time solving real problems—instead of cleaning up ones that started in the stockroom.
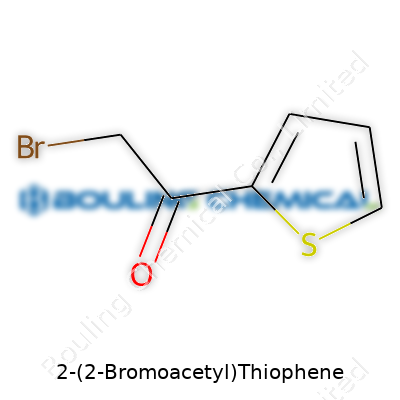
If you crack open any organic chemistry book, names like 2-(2-Bromoacetyl)thiophene sound downright intimidating. Strip away the jargon, though, and the purpose becomes clear. Each part of the chemical name carves out a road map: a thiophene ring ties to a bromoacetyl group at the second position. That small swap can turn a bland molecule into something special.
Chemists love numbers. For this compound, the chemical formula reads C6H5BrOS. If you’re mixing solutions or running a synthesis, that tally of carbons, hydrogens, bromine, oxygen, and sulfur tells you exactly how much ingredient to scoop out. Its molecular weight clocks in at 221.07 g/mol. Most labs rely on that figure—whether you’re scaling up a reaction or calibrating instrumentation—because getting it wrong leads to all sorts of headaches, from wasted reagents to flawed results.
I remember running into trouble during my own undergrad days. Calculating yields in organic labs felt tedious at times, but the lesson stuck: there’s no cutting corners. Using a slightly off molecular weight for a compound like 2-(2-Bromoacetyl)thiophene could mean doubling your error margin, especially in bigger batches. Pharmaceuticals, for example, can’t risk dosing errors. Schools could hammer this lesson a little harder. You learn fast that those decimal points aren’t trivial.
Precision becomes even more critical as research goes advanced. Bromine brings density and reactivity, which makes this compound a popular intermediate in pharmaceuticals and agrochemicals. Pairing it with the thiophene ring provides sites for future reactions, turning this little molecule into a Swiss army knife for chemists. But mess up the math, and every step after snowballs in the wrong direction.
Mistakes in chemical identification don’t just stay bottled up in labs. Sourcing the wrong compound or using a poor-quality reagent wastes time, drains budgets, and sometimes requires chucking whole batches. It also hits home in environmental chemistry. Disposing of misidentified or miscalculated chemicals adds avoidable strain on waste management, opening up possible ecological risks.
There’s also a safety angle. Combining chemicals with unexpected weights or formulas can introduce toxic byproducts or even cause explosions—everyone in a lab worries about that. With 2-(2-Bromoacetyl)thiophene, the bromine centre means you need full awareness of its proportions. OSHA and similar groups demand tight documentation for a good reason.
Improving database access in educational settings could help. Most students wrestle with outdated or paywalled chemical catalogues. Simple, open-source lookups—especially those spelling out structural diagrams and calculated weights—would let more people see the nuts and bolts of these molecules. Open collaboration between academic labs and web developers could shape a new norm.
Some labs have started integrating QR codes on reagent bottles that scan out chemical formulas, structures, and safety information straight to your phone or laptop. That beats guessing or rushing a calculation. It also gives a backup trail for quality control and inventory.
Direct, reliable information about things like 2-(2-Bromoacetyl)thiophene’s molecular weight and formula pays off far beyond neat notebooks. It supports safer experiments, better data reliability, and solid results, whether you’re building new medicines, greener pesticides, or just chasing the next breakthrough in your own small corner of the lab.
Anyone who’s spent time in a chemistry lab knows the dance between curiosity and practicality. You discover a compound, you see its promise, then you hit the wall: can you get enough of the stuff to actually run your project? It’s no different with 2-(2-Bromoacetyl)Thiophene. On paper, it’s a handy intermediate, especially for folks working on pharmaceuticals or specialty materials. The real question is, can you get your hands on it in quantities bigger than a coffee scoop?
Some big-name chemical suppliers list this compound. If you poke around, there are small glass bottles labeled with its name sitting on warehouse shelves in Europe, North America, and China. Those quantities — 1 gram, 5 grams, sometimes 25 grams — are great if your whole project fits on a lab bench. Need several kilos? Now you’re into back-and-forth emailing, negotiations, and probably a bit of suspense.
I once spent weeks hounding suppliers just to get consistent information on larger orders. Half the time, the response is a polite version of “maybe, let me call my cousin’s friend.” The other half, you see astronomical prices with no promise on lead times. Back to the drawing board for many projects. This issue stands out most for small companies, university groups, or anyone without the kind of cash flow or reputation that demands attention.
Suppose you find someone who can swing a deal for 10 kilos. Next, you cross the swamp of documentation. Even common organic intermediates can trigger alerts in customs. If your paperwork isn’t flawless or if there’s just a language barrier on an invoice, the shipment gets stuck. Don’t forget those extra fees for hazardous materials, temperature control, or re-packaging.
Pricing says a lot here. Small quantities fetch retail rates — $150-300 for a few grams. Bulk orders can drop the cost per kilo sharply, but only if you rally several buyers together or already buy thousands of different chemicals every year. Otherwise, you’re at the mercy of whoever decides to handle the paperwork.
Many cutting-edge syntheses grind to a halt not for lack of creativity, but because key reagents only trickle in small bottles. Companies and labs that want to move research into pilot scale or even low-volume production hit the same snag. In my own work, some ideas never left the notebook because intermediates disappeared from every catalog or came with minimum orders so high they drowned any small project.
It’s not just an administrative headache. If scientists and engineers can’t get what they need in bulk, progress gets stifled. Investors hesitate, projects stall, students get told to “pick something else.” Basic research and startup innovation end up in a holding pattern, not on purpose, but because the chemical logistics chain still hasn’t caught up with modern demand.
There’s room for improvement. I’ve seen some suppliers pool orders across institutions, cutting down costs and shipping headaches. More established companies sometimes share excess stocks, though these are the exceptions. Digital marketplaces are slowly making bulk transactions less mysterious, letting smaller labs piggyback on bigger deals. If manufacturers open up a bit and clarify minimum quantities, research at many places could move faster. Until then, anyone chasing 2-(2-Bromoacetyl)Thiophene at scale learns to expect some hunting, haggling, and improvisation along the way.
2-(2-Bromoacetyl)thiophene might sound like another obscure chemical, but it enjoys a bit of respect in many labs and specialty industries. Anyone who’s spent time in a synthetic chemistry lab knows that certain building blocks pop up again and again, and this one lands on the bench of those chasing new organic molecules. Its structure—a thiophene ring bearing a bromoacetyl group—packs a good deal of reactivity, which often draws synthetic chemists for its versatility in the hunt for more complex compounds.
My own brush with 2-(2-Bromoacetyl)thiophene came during a summer stint in a research lab, where it turned up in projects focused on small-molecule drug development. Medicinal chemistry labs use it to introduce thiophene units into drugs, helping shape how a molecule interacts with biological targets. The bromoacetyl group is just reactive enough to make further transformations pretty reliable. Route planning in pharma demands selectivity—getting just the right change at just the right atom—and this compound has an edge for those selective substitutions.
Organic synthesis isn’t just about drugs. These days, electronics and materials science are hungry fields for new, customizable molecules. Thiophene derivatives play a big role in organic semiconductors, light-emitting diodes (OLEDs), and solar cells. Chemists often use 2-(2-Bromoacetyl)thiophene when making building blocks for those materials. Swapping out that bromo for other groups really lets you tweak electronic properties. In short, it gives scientists a flexible launchpad when fine-tuning molecules for conductivity or light absorption.
The search for new reactions in organic chemistry often lives and dies by the raw tools on hand. 2-(2-Bromoacetyl)thiophene brings a rare mix—its thiophene ring resists breaking down too easily, and the bromoacetyl chain brings just enough “handle” to grab onto during a reaction. Research into new ligands, catalysts, and bioactive molecules benefits from this. In academic settings, you might see teams running parallel syntheses, swapping in this or that building block. The bromoacetyl group can react with nucleophiles, lending itself to countless modified products that wind up in reaction libraries for screening.
Synthesis with this compound isn’t trouble-free. That bromoacetyl group, for all its usefulness, can trigger unwanted side reactions if a chemist isn’t careful. Even students run into issues with stability or tricky purification steps. Streamlining reactions by fine-tuning solvents, temperatures, or protecting groups keeps things manageable, but isn’t always foolproof. More reliable procedures and some creative troubleshooting—think better purification columns or greener solvents—help nudge research along without burning through budgets or making too much waste.
Safety also presses on researchers working with bromo compounds. Anyone handling them needs good fume hoods and strong knowledge of their hazards. Training staff to spot exposure signs, and sticking to regular monitoring, cuts down on risk. Sharper oversight and clear protocols, in my own experience, kept incidents low even for newcomers in academic labs.
2-(2-Bromoacetyl)thiophene stays relevant by being adaptable. Its value across drug labs, electronics material development, and academic research isn’t just based on what it’s done already, but the doors it opens for new chemical frontiers. Investing in safer practices and more streamlined use makes sense for both productivity and people on the job.
| Names | |
| Preferred IUPAC name | 1-(Thiophen-2-yl)-2-bromoethan-1-one |
| Pronunciation | /tuː bromoʊ əˈsiːtɪl θaɪ.əˌfiːn/ |
| Identifiers | |
| CAS Number | [**'13615-11-3'**] |
| Beilstein Reference | 1201816 |
| ChEBI | CHEBI:88535 |
| ChEMBL | CHEMBL3633239 |
| ChemSpider | 81614 |
| DrugBank | DB08352 |
| ECHA InfoCard | 08f24e43-88f9-3a6c-b83a-54dff20be006 |
| EC Number | 617-734-2 |
| Gmelin Reference | 83952 |
| KEGG | C19108 |
| MeSH | D017937 |
| PubChem CID | 12514292 |
| RTECS number | KH8585000 |
| UNII | P4OM232SCD |
| UN number | UN2810 |
| CompTox Dashboard (EPA) | DTXSID70938666 |
| Properties | |
| Chemical formula | C6H5BrOS |
| Molar mass | 221.09 g/mol |
| Appearance | light yellow to yellow liquid |
| Odor | Odorless |
| Density | 1.699 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.4 |
| Vapor pressure | 0.0191 mmHg at 25°C |
| Acidity (pKa) | 9.31 |
| Basicity (pKb) | Basicity (pKb): 7.86 |
| Magnetic susceptibility (χ) | -80.98·10^-6 cm³/mol |
| Refractive index (nD) | 1.600 |
| Viscosity | Viscous liquid |
| Dipole moment | 3.5093 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 359.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | No ATC code |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06, GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P280, P305+P351+P338, P304+P340, P312 |
| NFPA 704 (fire diamond) | 2-(2-Bromoacetyl)Thiophene NFPA 704: 2-2-0 |
| Flash point | 101°C |
| NIOSH | PY8060000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 1 mg/m3 |
| IDLH (Immediate danger) | NIOSH: Unknown |
| Related compounds | |
| Related compounds |
Thiophene 2-Acetylthiophene 2-Bromothiophene 2-(2-Chloroacetyl)thiophene 2-(2-Iodoacetyl)thiophene |