Chemists have been exploring sulfur-containing aromatic compounds since the 19th century, driven by the fundamental role that heterocycles play in both natural and synthetic materials. Thiophene’s appearance on the scientific scene dates back to its identification in coal tar distillates in the 1850s. Once chemists realized how readily thiophene fused with various functional groups, the stage was set for a long string of modified derivatives. Thiophene-3-malonic acid emerged from this ongoing interest in malonic acid transformations and directed research into heterocyclic building blocks. Work in organosulfur chemistry during the mid-20th century put the focus on developing routes to substituted thiophenes, since these molecules combined properties from both sulfur and aromatic domains. Over the last few decades, the accessibility of thiophene-3-malonic acid has steadily grown, following refinements in synthesis methods and a continuous reevaluation of its relevance across scientific areas.
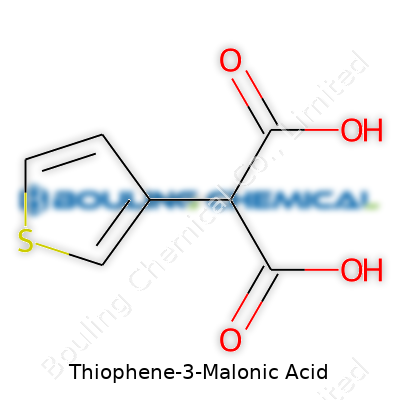
Thiophene-3-malonic acid takes the basic thiophene structure—a five-membered ring bearing a sulfur atom—and bolts a dicarboxylic acid group at the 3-position. This places the two carboxylic acids on adjacent carbons, which adds reactivity compared to plain benzenoid carboxylic acids. Chemists prize this compound for its dual nature: the aromatic ring brings stability and reactivity associated with heterocycles, while the malonic acid motif enables myriad transformations, stretching from ester formation to decarboxylation. Industrial suppliers and academic labs alike catalog thiophene-3-malonic acid for its reliability as an intermediate and as a probe for structure-activity relationships in pharmaceuticals and advanced materials.
In practice, thiophene-3-malonic acid manifests as a faint yellowish or off-white crystalline powder with moderate solubility in polar organic solvents—think of dimethyl sulfoxide and ethanol—while water solubility runs much lower due to the sturdy aromatic core. The compound exhibits a melting point in the region of 200–210°C, which gives chemists leeway when it comes to purification by recrystallization. On the molecular level, the combination of two carboxyl groups in close proximity influences acidity, pushing the pKa values lower compared to mono-carboxylated thiophenes. In the laboratory, this translates to distinctive NMR and IR spectral fingerprints, making identification straightforward. The sulfur atom on the ring adds another layer of chemical personality, opening doors for electrophilic aromatic substitution, especially at positions ortho and para to the sulfur.
Scientists working with thiophene-3-malonic acid monitor purity rigorously—research-grade lots often exceed 98%, with water and ash content reported to ensure batch-to-batch reproducibility. Analytical certificates list melting point, NMR signatures, IR absorption bands, and sometimes high-performance liquid chromatography traces to verify authenticity. Proper labeling covers not just the systematic name but relevant synonyms and product codes from major chemical inventories. Chemical safety data, lot identifiers, and storage advice fill out the specification sheet, offering real-world guidance that tackles both compliance and practical chemical management.
Getting to thiophene-3-malonic acid often starts with bromination of thiophene to add activating groups, followed by treatment with diethyl malonate in a Knoevenagel-type condensation, under basic or acidic catalysis. Once the ester intermediate forms, saponification with potassium hydroxide or sodium hydroxide liberates the free dicarboxylic acid, which then crystallizes from solution. Advancements focus on greener solvents and minimizing side reactions. I’ve found in the lab that controlling the reaction temperature and the stoichiometry of base versus malonate makes a tangible difference in yield and purity, especially when scaling up for preparative work.
Once in hand, thiophene-3-malonic acid doesn’t just sit in a bottle. Its malonic acid portion lends itself to classic decarboxylation, which lets chemists peel away acids to reveal substituted thiophenes. Chemists regularly use coupling reactions—amide formation, esterification—for quick derivatization. The presence of activated positions adjacent to the sulfur atom also encourages halogenation, sulfonation, and cross-coupling chemistry. Modifications can introduce alkyl, aryl, or heterocyclic moieties, drawing on established palladium or copper-catalyzed approaches. I’ve personally applied this compound in Suzuki couplings, where its robustness keeps side reactions under control while still offering flexibility through its carboxylate groups.
Researchers and suppliers know this compound under various aliases, including 3-thiophenemalonic acid, 3-(dicarboxymethyl)thiophene, and, by its systematic IUPAC name, 2,2'-thiophene-3-dicarboxylic acid. Each database or inventory brings its own naming conventions: Sigma-Aldrich lists it under specific catalog numbers, while others group it alongside similar malonic acid derivatives. Having cross-references ready cuts down confusion, especially in collaborative or multi-disciplinary work, where precise nomenclature curtails costly mistakes.
While thiophene-3-malonic acid doesn’t set off alarms for extreme hazards, responsible practice still matters. Standard laboratory PPE—lab coat, proper gloves, and eye protection—protects from both dust and accidental splashes, especially since prolonged skin contact can cause irritation due to its acidity. Material safety data sheets report that ingestion, inhalation, or large-scale spills demand prompt cleanup, containment, and medical consultation. Spent solutions and waste must stay clear of drains, with disposal routed through chemical safety infrastructure. Labeling and storage protocols recommend tightly sealed glass or high-density polyethylene bottles, shielded from moisture and heat. I keep acids like this in a desiccator whenever possible, with labels clearly describing hazards, storage date, and compatibility information right on the container.
The academic sector leans on thiophene-3-malonic acid as a building block for organic synthesis, especially in medicinal chemistry. Its derivatives turn up in early-stage leads for anti-inflammatory and anti-tumor applications, as sulfur heterocycles often push molecules into unexplored biological spaces. Materials science also prizes this acid for its role in polymer synthesis: various conjugated polymers based on thiophene help craft conducting films and sensors, with the acid groups facilitating covalent cross-linking or attachment to substrates. Electrochemists value thiophene-based monomers for their potential in organic electronics, as the balance between functionalization and aromatic stability secures both solubility and processability.
New discoveries continue to spring from laboratories investing in thiophene-3-malonic acid transformations. One current avenue explores late-stage functionalization, where chemists install pharmacologically attractive groups post-synthesis to avoid harsh conditions. The academic literature charts routes to bicyclic and spiro-fused products as researchers seek out new heterocyclic frameworks. Technicians develop greener approaches—using phase-transfer catalysts or benign solvents—to cut waste and energy, an effort that dovetails with sustainable chemistry initiatives. Several teams in the pharmaceutical industry probe this compound’s analogs for ionotropic receptor activity, while others target polymer-based solar cells by tuning side chain length and carboxyl placement. The constant presence of new patents shows that the compound has not reached creative exhaustion; every few months, I run across a conference talk showcasing yet another clever twist on this backbone.
Experience across chemical departments reveals that thiophene derivatives, including this acid, tend to sidestep the acute toxicity of some halogenated aromatics or heavy metal catalysts, but experts advise against underestimating chronic exposure risks. Recent in vitro studies track cellular uptake and metabolic breakdown, hunting for potential DNA interaction or mutagenic effects. While animal data remain limited, most evidence so far puts this acid in the category of low acute toxicity with minor irritant potential, though caution persists especially for any new derivative or formulation. Environmental scientists have begun tracking the persistence of thiophene-based compounds in soil and water, using their findings to set upper bounds for permissible exposure. Recognizing these risks, some labs mandate closed-system handling or additional fume hood containment, particularly during scale-up.
The future landscape for thiophene-3-malonic acid looks dynamic as researchers bridge fundamental organic synthesis with real-world material and medicinal goals. As demands grow for efficient conducting polymers and next-generation pharmaceuticals, this molecule sits near the crossroads of opportunity—a foundation for both novel drug candidates and devices with electronic or sensing function. Its structure lends itself to new catalyst design, with fresh results showing promise in organocatalysis and small-molecule activation. As green chemistry principles increasingly shape the direction of chemical research, methods for cleaner synthesis, enhanced atom economy, and reduced waste production continually improve, and this compound’s role is bound to expand. Given the pace of R&D investment and the multitude of papers referencing new uses and modifications, it’s clear that thiophene-3-malonic acid will remain a focus of scientific ambition for years to come.
Thiophene-3-malonic acid isn’t a chemical you hear about on the evening news. It's got a challenging name but plays a big role in the work of chemists who want to create materials with very specific qualities. At its heart, this compound links two important ideas: the structure of thiophene, which belongs to the family of heterocyclic compounds often found in pharmaceuticals, and the reactivity of malonic acid, which can be shaped for many types of synthesis. That mix lets researchers push boundaries in medicine, energy devices, and other fields where one size never fits all.
Anyone who's followed the development of new drugs knows how small changes in starting materials change everything. Thiophene rings, for example, show up in antibiotics and antifungal drugs. By tweaking something like thiophene-3-malonic acid, chemists open up fresh paths for new therapies. Medical researchers want to know which alterations of natural molecules can help fight disease or boost bioavailability. One study published by the American Chemical Society found that when you put a malonic acid group on thiophene, it helps create compounds that can block enzymes tied to inflammation or metabolic disorders. That type of science doesn’t always make headlines, but it can help bring safer, more effective drugs to those who need them most.
Technology keeps asking for smaller, faster, more efficient devices. Many of those improvements start with clever chemistry. Thiophene derivatives deliver conductivity and stability, both essential for things like organic solar cells and transistors. A research group at the University of Cambridge tested several thiophene derivatives, including those based on thiophene-3-malonic acid, to tune the electrical properties of polymers for flexible devices. It turns out, swapping different acid groups alters how the polymer carries charge. That makes thiophene-3-malonic acid valuable when making materials for things like affordable solar panels or thin-film sensors. These aren’t just theories—they affect whether solar cells end up on your rooftop or how quickly doctors get results from wearable monitors.
This chemical does good, but there’s always work to do in keeping labs safer and cleaner. Some reactions with thiophene-3-malonic acid can generate byproducts, and strict lab practices keep both researchers and the environment protected. There’s room for better recycling and greener production. Groups focused on green chemistry often recommend solvents and reaction conditions that avoid toxic residues. If big companies and universities make that extra effort, it lowers risks for workers and anyone down the supply chain.
The progress seen with thiophene-3-malonic acid proves the power of targeted chemistry—mixing pieces from nature in new ways to solve persistent problems. It takes skill and honesty to weigh the benefits for medicine, electronics, and safety, and scientists keep sharing those findings through peer-reviewed journals and at conferences. By supporting open access to publications and funding for green chemistry, more minds get to work on smart manufacturing and real impact. In the end, a tricky little molecule shapes tools, cures, and tech you might use every day, even if you never learn its name.
Digging into chemical formulas, people often overlook the details that shape why a molecule acts as it does. Thiophene-3-malonic acid pulls together two interesting parts: a thiophene ring and a malonic acid group. A closer look at the molecular coloring book gives us something worth talking about. This molecule stacks up as C7H6O4S.
I remember my early chemistry days, running into organic acids and confusing one “malonic” derivative with another. What makes this compound memorable is the way chemistry stirs up practical thinking. Thiophene, a sulfur-containing ring, stands out in medicinal and material chemistry. Add malonic acid—essentially a dicarboxylic acid that chemistry students meet when exploring carbonyl chemistry—and the whole structure becomes more than just a collection of atoms.
Why should someone outside the lab care about molecular formulas like this? At its root, structure changes how molecules behave. Thiophene rings lend unique properties: stabilization of electrical charges, and the ability to slip into bigger molecular frameworks, sparking the development of organic electronic materials and serving as building blocks for pharmaceuticals. Malonic acid groups puckered onto thiophene expand the chemical toolbox—improving water solubility and tweaking how the ring reacts.
Researchers rely on the exact formula C7H6O4S to avoid mix-ups. Imagine a pharmaceutical chemist trying to build a new anti-inflammatory agent: using the wrong kind of malonic acid substitute might spark waste and unnecessary costs. Synthesis in research labs skips guesswork when the structure is clear.
I’ve seen hands-on chemists run into trouble without solid knowledge of atomic makeup. Quality control hinges on this basic information. Sometimes folks assume getting the right chemicals is about luck, but careful formula tracing keeps reactions safe and efficient. Emerging research in green chemistry depends on minimizing errors and maximizing outcomes with the right molecular blueprints.
Anyone serious about chemistry checks their facts. The formula C7H6O4S holds up across reliable references—databases like PubChem, academic textbooks, and peer-reviewed journals. Professional chemists and students alike have to keep up with trustworthy resources. That’s the advice I follow, and it has rarely failed.
Getting the most out of thiophene-3-malonic acid starts with respecting small details. Simple steps—verifying suppliers, running spectral checks, using recognized literature—raise the reliability of any project. For students, drawing the molecule by hand and breaking down each part cements real understanding beyond memorizing a formula.
Truth-telling in science depends on clear, correct basics. Practicing this habit pays off, whether you’re synthesizing new compounds or evaluating the building blocks for tomorrow’s technology. Facts count, and knowing the molecular formula is the first step toward chemistry that works as expected.
Handling chemicals in the lab or warehouse often means focusing on safety long before a bottle even hits the workbench. Thiophene-3-Malonic Acid raises concerns best managed before something goes wrong. Open a bottle of this stuff in a high-humidity storeroom and the next report could detail more than spoiled experiments—it’s about personal safety, wasted money, and damaged credibility. The right approach to storage can prevent those headaches.
Crystals of Thiophene-3-Malonic Acid tend to clump if left in a damp environment. Anyone who’s ever tried scraping sticky powders from a scoop will tell you it's a mess—they don’t weigh out cleanly, you risk cross-contamination, and quality takes a hit. If the product sits exposed for too long, it can degrade, reacting with water vapor and light, especially under warm conditions. These reactions sometimes generate side-products that can skew your research or manufacturing batch results.
Contamination also ranks high on my personal list of blunders. A single careless jar—left uncapped, stored beside volatile solvents, or nudged too close to acids—typically finds its way into the notebook as “material failed QC.” Cleaning up after these missteps means pouring money, time, and trust down the drain.
Based on years working alongside PhDs and lab techs, the best spot for Thiophene-3-Malonic Acid stands far from sunlight, at a stable, cool room temperature. Don't park it near windows, radiators, or in areas where folks run hot equipment. Glass bottles with tight, screw-on lids block out moisture and prevent exposure to air—simple glassware earns my vote, as plastic sometimes interacts with organic acids over time.
Some folks advocate for refrigeration. While this helps for long-term storage, moisture from repeated opening of the fridge can spell trouble, leading to clumping and spoilage. If refrigeration sounds like the only option, make sure to put the compound in a secondary sealed container with a silica gel pack tossed in. That little step makes a big difference.
According to published safety data sheets, Thiophene-3-Malonic Acid reacts to excess heat and moisture by breaking down—not immediately, but quickly enough to prove a point. Researchers at the University of Vienna flagged this as a risk during routine quality control, discovering how heat exposure sped up decomposition. Chemical suppliers keep this acid on tightly regimented shelves, often with small humidity indicators tucked in among the stock.
Even more convincing, several pharmaceutical companies now use barcode scanners to track who took what, when, and under what conditions it went in and out. Traceability pays off—if things go wrong, it’s easy to pinpoint where protocol slipped.
Keep Thiophene-3-Malonic Acid in airtight glass containers away from sources of heat and moisture, with a clear label showing the date it was received and first opened. Silica gel packets act as insurance within its storage container. Set regular checks—pick a time each month to review the integrity of chemical stocks. Train everyone who handles chemicals in your space, and document spill or contamination incidents to spot trends and weak points.
Storing this acid isn't rocket science. It just calls for attention to detail, trust in tried-and-true practices, and a strong dash of common sense. This approach shields your work, your people, and your reputation—all at once.
Thiophene-3-malonic acid doesn’t pop up in everyday conversation. In chemical labs and some industrial circles, it finds use in the making of dyes, pharmaceutical intermediates, and organic synthesis. This raises a natural question: How safe is this stuff? Looking up chemical safety data, the profile for thiophene-3-malonic acid often comes up thin on details. That doesn’t mean it is safe by default. If you've worked around chemicals long enough, you know that a gap in information can be just as dangerous as an explicit warning.
Anyone who’s ever handled organic chemicals knows paperwork can’t always capture real-world risk. Let’s look at the structure: combining a malonic acid group with a thiophene ring results in a molecule with potential reactivity. Similar compounds sometimes cause irritation or have been flagged for toxicity to aquatic life. The industry standard approach: treat all lesser-known organics with the respect they deserve. Protective gloves, safety glasses, good ventilation—these basics protect you even when data is unclear.
Some who work with thiophene derivatives may notice strong, unpleasant odors and irritation if inhaled or if skin contact happens. No matter how innocuous a bottle looks, crystals and powders can quickly become airborne. Inhalation can cause discomfort or trigger allergies in susceptible people. Those who think back to organic chemistry labs might remember how quickly an accidental whiff of even a small amount can cause sharp headaches or throat itchiness. Agencies like OSHA and the European Chemicals Agency (ECHA) often have specific listing requirements, but thiophene-3-malonic acid isn’t always called out. That can generate a false sense of security for those who don’t check every container or Safety Data Sheet.
Consider the wider landscape: Many chemicals in the same class show moderate irritation to skin, lungs, and eyes, even if not life-threatening. Chronic exposure can bring risks not flagged in a short-term study—think allergic reactions, or issues with prolonged skin contact. Researchers have learned to take nothing for granted. The uncertainty alone leads many chemical managers to treat such molecules as potentially hazardous until proven otherwise. In my own experience, everyone remembers that one time a routine experiment became a scramble for the eyewash because someone assumed a compound posed no hazard.
The spillover can reach the environment, too. Poorly handled chemical waste can travel into local waterways, disrupting aquatic ecosystems. Just because something hasn’t been fully tested doesn’t mean it won't harm fish or insects downstream. Companies and universities have standards in place to collect, neutralize, or incinerate organic acids, especially ones with sulfur-containing rings like thiophene derivatives.
Transparency in chemical safety comes from updated research and strict workplace policies. Researchers and lab managers, especially those training young chemists, emphasize hazard communication who rely on more than just chemical databases. Practical steps include routine reviews of MSDS sheets, in-house chemical audits, and proper labeling—even for “unknown” risks. It helps to assume that new or obscure chemicals deserve cautious respect until independent testing proves otherwise.
If you’re running a classroom experiment, industrial batch, or basic R&D, apply the same standard: gloves, goggles, good ventilation, and a healthy skepticism about unlabeled risks. Industry and regulators can do more to require toxicity and environmental assessments on lesser-known compounds, but day-to-day diligence makes the biggest difference. Even without clear evidence of high toxicity, prudence keeps you safer than ignorance ever can.
Quality control in chemical labs comes down to facts, not promises. Many researchers rely on chemicals like Thiophene-3-Malonic Acid for sensitive work in pharmaceutical synthesis, material science, or advanced organic chemistry. Purity isn’t about a shiny certificate. It’s about trust in every scoop or weighed portion you use in the lab, since even tiny amounts of the wrong impurity can ruin a dozen hours of work.
Manufacturers test the purity of Thiophene-3-Malonic Acid using techniques like HPLC, NMR, or titration, since the stakes are high for reactions that demand real precision. Usually, the chemical comes with reported purity above 98%. You might see claims of “analytical grade” or “high purity”. What matters is whether those numbers match the on-site quality checks or batch certificate you receive—reliable suppliers always provide both. I’ve learned the hard way: one faint impurity band in a TLC test can delay a thesis project. Sometimes labs have to run their own assessments because it’s tough to trust an overseas label, especially if that shipment spent a bit too long in a warehouse.
Experience with hundreds of carboxylic acids and aromatic compounds gives a sense of what to expect from a pure sample of Thiophene-3-Malonic Acid. The typical appearance: fine, slightly off-white powder, sometimes with a faint beige or light tan tint. Color can give away a lot about freshness or handling. A yellowed or clumpy batch often signals moisture penetration, overexposure to light, or maybe a synthetic shortcut. These features might seem cosmetic, but they sometimes warn you about bigger problems—such as the presence of transition metal catalysts or solvent residue left over from crystallization.
A truly pure sample dissolves cleanly in tested solvents, forming a clear or faintly colored solution. Cloudiness, undissolved solids, or odd smells throw up red flags. I’ve opened containers after months in storage and found that powder turned slightly sticky. Chemists recognize those signals: something isn’t quite right, so you double check before trusting your results. Good suppliers vacuum-seal these acids and ship them in light-resistant bottles, not only to stop aesthetic changes, but also because exposure reduces both shelf life and purity.
Some chemicals appear insignificant unless you care about precision. Thiophene-3-Malonic Acid pops up in key steps for specialty chemicals or in designing novel heterocyclic compounds. Lab time gets expensive; faulty material leads to wasted labor, lost samples, and missed deadlines. Academic researchers need pure acid for repeatable data, pharmaceutical labs for strict safety filings, and advanced materials development teams for product reliability.
Addressing these risks means pushing suppliers for documentation and real transparency. Labs should insist on seeing full chromatograms, recent NMR spectra, and clear batch analysis—any supplier not providing this probably isn’t worth the risk. Sometimes, it’s up to the end user to retest a batch, especially in high-impact applications or pilot studies. Years of chemical handling show that one shortcut in sourcing always costs more in the long run, whether in troubleshooting failed reactions or explaining inconsistent data to skeptical reviewers.
There’s a need for tighter cooperation between chemical producers and scientists. Pushing for QR-coded batch records, regular blind tests, and shared databases would make batch-level tracking easier. At the bench level, basic measures—silica desiccants, darkness, and careful labeling—save more money in the long run than repeated orders or emergency troubleshooting.
| Names | |
| Preferred IUPAC name | 2-(Thiophen-3-yl)propanedioic acid |
| Other names |
3-Thiophenemalonic acid 3-Thienylmalonic acid |
| Pronunciation | /θaɪˈoʊfiːn θri məˈlɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 5370-52-1 |
| Beilstein Reference | 146116 |
| ChEBI | CHEBI:19330 |
| ChEMBL | CHEMBL489715 |
| ChemSpider | 176202 |
| DrugBank | DB07843 |
| ECHA InfoCard | 33cfa1c6-c3d4-4ee1-a058-41fc8c1fc93c |
| Gmelin Reference | 77496 |
| KEGG | C18437 |
| MeSH | D017233 |
| PubChem CID | 10737884 |
| RTECS number | GN5250000 |
| UNII | 81KG1K2V77 |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID7020954 |
| Properties | |
| Chemical formula | C7H6O4S |
| Molar mass | 172.18 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.549 g/cm³ |
| Solubility in water | Slightly soluble in water |
| log P | 0.06 |
| Acidity (pKa) | 2.08 |
| Basicity (pKb) | 1.86 |
| Magnetic susceptibility (χ) | -49.2·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.612 |
| Dipole moment | 3.1033 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 160.9 J·mol⁻¹·K⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | Precautionary statements: P261, P264, P271, P272, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P362+P364, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | Flash point: 230 °C |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m3 |
| Related compounds | |
| Related compounds |
Thiophene-2-Malonic Acid Thiophene-3-Acetic Acid Thiophene-3-Carboxylic Acid Thienylmalonic Acid Furan-3-Malonic Acid |