People began looking into thiophene derivatives back in the late 1800s, driven by curiosity about sulfur-containing heterocycles. Early synthetic chemists noticed thiophene rings popping up as impurities in coal tar and other industrial feedstocks. By the mid-20th century, efforts to tweak the thiophene core led to compounds like thiophene-2-ethylamine, which pairs an amine side chain with the core heterocycle. Over the decades, labs chasing new pharmaceutical and agrochemical scaffolds experimented with this structure. As I’ve seen in research archives, simple modifications on a heterocyclic ring can spark entire research veins, yielding compounds that sometimes make a mark in the real world.
Thiophene-2-ethylamine stands out as a sulfur-containing aromatic amine, prized in organic synthesis and downstream chemical development. With its structure composed of a 2-ethylamine substituent off a thiophene ring, the molecule brings chemical reactivity that attracts chemists designing target molecules for drug discovery or materials development. In real-life chemical labs, team members reach for this building block when they’re after nitrogen and sulfur together in a compact framework. The amine group on a heterocycle isn’t just a footnote—it gives scientists a foundation for further functionalization.
In standard conditions, thiophene-2-ethylamine appears as a colorless to pale yellow liquid. It gives off a distinct odor—anyone who’s spent time around amines and thiophenes knows the sharp, sometimes slightly fishy note. Physically, the compound boasts a moderate boiling point, somewhere between 198°C and 202°C, so it does not evaporate readily at room temperature. Its density hovers near 1.08 g/cm³. The molecule dissolves well in common organic solvents such as ethanol, chloroform, and ether. Chemically, the amine group offers nucleophilicity, and the thiophene ring can stabilize charges, which makes the structure versatile. It combines two functional handles in one relatively small molecule—chemists value dual-purpose functionality like this in synthetic routes.
Producers specify purity by gas or liquid chromatography, typically offering thiophene-2-ethylamine at 98% or higher. Labels indicate boiling and melting points, flash point, solubility, and storage precautions. As a moderately hazardous substance, vials come marked with hazard pictograms required for amines: “Irritant” and, for some jurisdictions, “Harmful if inhaled.” Labels include the CAS number (104-81-4) and structural details for inventory and regulatory tracking. Specifications reflect real-world needs in labs, where shelf life, impurity levels, and batch consistency count just as much as chemical identity.
The most common route relies on nucleophilic substitution. Chemists start from 2-bromothiophene and react it with ethylamine under pressure or in a sealed tube, sometimes using copper catalysis to speed things up. The resulting mixture goes through purification steps like extraction, neutralization, and distillation. In my lab days, handling thiophene derivatives meant dealing with stubborn aromatics that resist substitution—catalysts and elevated temperatures help push the reaction along. Scaled-up versions may use flow reactors to control temperature and minimize exposure, supporting operator safety and reproducibility.
Once you have thiophene-2-ethylamine, the molecule is ripe for further transformations—the amine group enables acylation, alkylation, and even reductive amination. The thiophene ring tolerates electrophilic aromatic substitution, allowing introduction of various substituents at the 5-position. Coordination chemists, for example, attach metal centers to the nitrogen or sulfur, generating new complexes for catalysis studies. Medicinal chemistry teams often protect the amine with carbamate or sulfonamide groups to test its biological profile without rapid degradation. In hands-on research, this type of reactivity opens new doors in chemical biology and organic synthesis.
Thiophene-2-ethylamine goes by several names: 2-(2-thienyl)ethanamine, 2-thienylethylamine, and beta-thienylethylamine all refer to the same core structure. In vendor catalogs, you’ll sometimes spot it under names like T2EA or even just 2-ethylaminothiophene. The multiple names reflect the classic confusion in chemical trade and research—seasoned chemists double-check synonyms to avoid costly mix-ups during ordering or manuscript prep.
Dealing with thiophene-2-ethylamine involves handling like any small-molecule amine—ventilated fume hoods, nitrile gloves, goggles, and lab coats form the basic kit. Even though the compound doesn’t vaporize easily, its vapors irritate the respiratory tract and eyes. Spills produce stubborn odors that linger unless cleaned promptly. Waste disposal follows local environmental rules and hazardous waste protocols. Training workers on spill management, proper storage, and chemical compatibility reduces accident risks. As organizations like OSHA have found, consistent safety routines lower incidents in facilities that work with sulfur- and nitrogen-based chemicals.
In the chemical industry, thiophene-2-ethylamine finds use as a raw material for synthesizing advanced intermediates. Pharmaceutical researchers reach for it during lead optimization, building up libraries of analogs with diverse bioactivities. Material scientists sometimes experiment with it when developing sulfur-containing polymers or heteroaromatic dyes. Its use isn’t limited to basic research; agrochemical developers leverage the unique chemical space of thiophene rings linked to amines, hoping for new modes of action against pests or plant diseases. During my time consulting in specialty chemicals, I saw teams continually diversify their building blocks, looking to stay ahead in patent space by using non-obvious motifs like this one.
Ongoing R&D focuses on exploring new synthetic methods, such as green chemistry approaches or more efficient catalytic systems. Researchers analyze metabolic profiles, seeking clues about how small changes on the ring or side chain alter biological activity. Analytical chemists refine detection techniques—liquid or gas chromatography linked with mass spectrometry proves essential for tracking reaction progress and identifying trace impurities. Global supply chain pressures sometimes push manufacturers to look for sustainable feedstocks or lower-energy processes. The push for new applications in pharmaceuticals, materials, and diagnostics drives further investment in new analogs and data-driven structure-activity relationship studies.
Safety assessments reveal moderate acute toxicity if inhaled or ingested, with reports of mild liver stress in prolonged animal studies. For skin and eye contact, irritation turns up as the main concern. Long-term exposure limits lack precise definition, but precaution remains key. Research teams keep MSDS sheets updated and train staff on early recognition of overexposure symptoms. Risk management comes down to engineering controls, personal protective gear, and clear handling protocols. In academic settings where risk tolerance tends to run low, even low-dose exposures prompt close supervision, especially for new researchers just starting in the lab.
Looking ahead, new use-cases likely emerge as drug discovery and material science continue seeking fresh scaffolds. The modular nature of thiophene-2-ethylamine means researchers can plug this building block into high-throughput synthesis, generating large libraries for machine learning-guided screening. With green chemistry principles gaining ground, scientists may develop milder, more sustainable synthesis techniques that avoid hazardous reagents and excess waste. As regulatory environments tighten, documentation and supplier transparency increase in importance—the compound’s utility hinges on clear provenance and compliance. Over time, as new data emerges about biological or environmental impacts, the risk-benefit calculus may shift, but the versatility of thiophene-2-ethylamine ensures it stays relevant for years to come.
Thiophene-2-ethylamine is not a term that comes up in everyday conversation, but it has quietly found a place in chemical laboratories and manufacturing facilities across different fields. This compound, built around the thiophene ring, connects to an ethylamine side chain and opens doors for researchers working on new materials and pharmaceuticals. I remember first reading about it during a summer job in a university chemistry department. There, it showed up as an ingredient in the search for better-performing drugs and advanced polymers.
Drug development stands as one of the main areas where chemists pay attention to molecules like thiophene-2-ethylamine. Medicinal chemists sometimes need building blocks with specific characteristics, and the thiophene ring is known for bringing stability and reactivity. Studies highlight these properties, and the U.S. National Library of Medicine shows multiple research efforts using thiophene-based cores for therapy design.
It’s not that every new medicine uses this compound, but I’ve seen it referred to in research papers for early-stage work on treatments for central nervous system disorders and cardiovascular conditions. The presence of the thiophene group helps modify biological activity and can make a potential drug more effective or safer. Chemists appreciate these modifications and keep looking for ways to fine-tune the molecule for the best results.
Outside pharmaceuticals, thiophene-2-ethylamine finds a home in making specialty chemicals and intermediates. My experience with organic synthesis tells me that having a versatile molecule on hand saves time and money. Thiophene-2-ethylamine fits this bill, as the amine group easily binds to acids, aldehydes, or even isocyanates to produce a range of compounds.
Material science picks up this baton, with researchers combining thiophene-based units into polymers for electronic and sensor applications. The flexible structure forms part of conductive polymers that run in solar cells, LEDs, or flexible screens. Clean energy innovation depends on finding new materials that perform better, last longer, and cost less to produce. Thiophene derivatives play a growing role in meeting these goals.
Any discussion about chemicals should include honest talk about safety and regulation. I have seen situations where new compounds get flagged for potential misuse, or environmental impact, especially as industry regulations get stricter worldwide. Thiophene derivatives can act as intermediates for more potent substances, which means chemical suppliers need to keep close watch on who buys and uses them.
To build trust, manufacturers and academic institutions need to keep solid records, follow local and international law, and provide clear safety information. The European Chemicals Agency and the U.S. Environmental Protection Agency offer guidance on storage, labeling, and disposal. Taking these steps helps prevent accidents and chemical leaks, and protects workers and communities.
Innovation in chemistry keeps rolling as researchers search for new solutions in health and technology. Compounds like thiophene-2-ethylamine serve as stepping stones in this process. Thoughtful research, focus on safe use, and open sharing of new knowledge help unlock new benefits—without putting people or the planet at risk.
Ask any chemist about thiophene-2-ethylamine, and you’re likely to get a raised eyebrow. This isn’t one of those household-name compounds, but anyone working with chemicals in a lab or factory setting will run across tricky molecules just like this one. Sporting a thiophene ring and an ethylamine group, this organic chemical slips into research projects or crop protection formulations, often seeking a way to make reactions work smoother or help synthesize other substances. But what does it do to people who touch it—or even breathe in its fumes?
Start with the basics. Thiophene-2-ethylamine looks like a colorless to pale yellow liquid, and at room temperature, the stuff carries a pungent odor. Samplers who catch its smell often note burning eyes or mild cough, hinting that the vapor packs some punch on direct contact. Reports show skin irritation, redness, and even mild burns if you get careless with handling. Inhaling it for long stretches or in high concentration could invite headaches, dizziness, or nausea.
Animal testing and chemical structure both point in the same direction: here is a compound that shouldn’t be inhaled or splashed onto bare skin. Acute toxicity studies in animals (usually rats or mice) show that moderate exposures can knock out their liver or nervous system. The numbers back this up—LD50 values sit between 100 and 300 mg/kg in most reports, which makes the stuff quite a bit more hazardous than typical household cleaning agents.
Walk into a well-run synthetic organic lab, and you’ll see gloves, goggles, and lab coats—even when people aren’t dealing with the most notorious toxins. The trouble starts in shops or home labs where safety takes a back seat. Thiophene-2-ethylamine doesn’t have the explosive reputation of certain amines, but its liquid form means it splashes easily and evaporates, creating vapor that finds eyes and lungs. Respiratory protection makes sense. Chemical fume hoods and closed reaction vessels prove their value every day in workplaces I’ve seen.
OSHA doesn’t publish a specific permissible exposure limit for thiophene-2-ethylamine, but companies using it often impose their own internal exposure ceilings. These decisions draw on the general culture—if a substance causes clear irritation or carries a moderate toxicity profile, staff get trained in spill cleanup, ventilation, and first aid.
Disposing of thiophene-2-ethylamine brings its own layer of complication. Pouring it down the drain isn’t just lazy; it sets up groundwater risks and breaks local chemical waste laws. Waste disposal firms typically handle amines and sulfur compounds as hazardous waste. Municipal treatment plants aren’t designed for this class of molecules, so responsible users ship it out with a manifest and traceable paperwork. It’s about preventing soil and water contamination, but also about avoiding fines or worse.
Anyone using thiophene-2-ethylamine should start with the Safety Data Sheet and the wisdom of experienced chemists. Gloves, face shields, ventilation—the investment pays for itself every time someone leaves the lab at the end of the day without a medical incident. Employers can run short training sessions on emergency eyewash use, spill response, and labeling. In smaller research groups, a culture of peer review in each step creates checks that no corner gets cut.
Thiophene-2-ethylamine doesn’t make the evening news. The best way to keep it that way comes down to discipline, respect for unpleasant side effects, and a willingness to part with a little extra company cash for safe disposal.
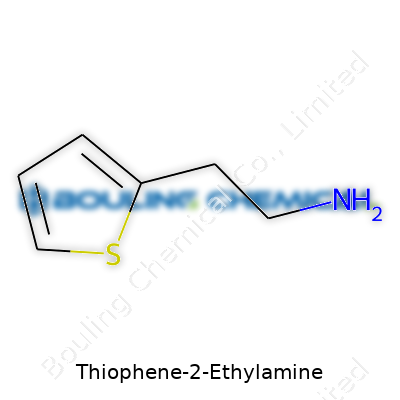
Thiophene-2-ethylamine, as the name hints, features a basic backbone borrowed from thiophene, a five-membered aromatic ring. Thiophene rings have four carbons and a sulfur atom sharing the spotlight, making them aromatic and giving a unique twist compared to more common carbocyclic rings. In thiophene-2-ethylamine, there’s an ethylamine group tacked onto the second carbon position of the ring. The chemical formula ends up being C6H9NS. That formula doesn’t just look good on paper—it packs meaning, especially for those in organic chemistry and pharmaceuticals.
A strong foundation in structural formulas has always made new research easier for me. Back during my lab days, I’d see that just a single substituent—like moving an amine group across the ring—could swing chemical properties and reactivity from night to day. With thiophene-2-ethylamine, the presence of the ethyl chain and an amine creates new opportunities for reactivity. Through simple trial and error on paper and in the lab, I learned this formula is more than a collection of letters and numbers: it signifies reactivity, possible interactions, and hints at biological activity that’s attracted a good deal of attention lately.
Thiophene derivatives bring serious value in electronics as well as in medical research. They slip into roles as intermediates for pharmaceutical compounds that scientists hope will manage everything from inflammation to neurological disorders. In those settings, accuracy with the chemical formula matters. I remember sifting through countless sample labels—knowing the difference between C6H9NS and similar isomers kept costly errors at bay. Industry professionals, whether designing new drugs or experimenting in synthetic routes, need precise details to keep research reproducible and products reliable. Getting the formula right isn’t trivia—it’s essential.
Every new amine-thiophene combination produces possibilities, but also risks. In pharmaceutical spaces, researchers must track toxicological profiles, as new amine compounds may interact unpredictably with enzymes or cellular receptors. Experience taught me to respect every new structure for its potential as well as its hazards. Chemicals with amine groups, including thiophene-2-ethylamine, sometimes trigger skin or respiratory irritation. Environmental disposal also turns into a challenge unless everyone along the chain knows what’s inside that small bottle. Mistakes in handling, mislabeling, or misunderstanding the basic chemical composition create real danger for everyone in the lab and beyond.
Accuracy and openness drive research in thr right direction. In my own work, I made a point to rely on validated sources—databases like PubChem and Sigma-Aldrich verify that thiophene-2-ethylamine is correctly noted as C6H9NS. These checks reinforce lab safety, facilitate clear communication, and prevent setbacks. Groups advancing new uses for these compounds—such as functionalized materials for electronics or potential therapeutics—benefit from sharing clear chemical information so others can reproduce results safely and effectively.
To get past industry pitfalls, education and continued professional development remain key. Chemists staying current with reference materials and practicing good labeling prevent mix-ups and save lives. Collaboration among manufacturers, academic researchers, and regulatory agencies enforces high standards. Responsible disposal protocols and honest communication work better than fancy safety posters or lectures alone. In real life, attention to basic chemical formulas helps build safer workplaces and keeps the public’s trust where it belongs.
Anybody who’s worked in a lab for a while knows how careless storage of chemicals can throw a wrench into daily work—and even more, risk people’s health. Thiophene-2-ethylamine isn’t something you just leave on a shelf and forget. Its chemical nature means it brings hazards if ignored: it can irritate your skin, may splash, and releases fumes that get into the air with little encouragement. You get careless, someone gets hurt, or expensive material vanishes. That’s never worth it.
Every bottle should have a clear label. Folks in labs sometimes assume everyone knows what’s where—until an intern grabs the wrong thing. A proper label cuts out guesswork, lowers accident risks, and lets emergency responders act fast if something spills or catches fire.
Glass bottles with tightly fitted caps stand up best against this chemical’s tendency to escape as vapor. Plastic can sometimes react with smells or liquids, which means tainted chemicals or—worst of all—a bottle that fails without warning.
Keep packs upright and out of sunlight. It’s tempting to squeeze extra bottles into bright, warm corners, but heat speeds up unwanted reactions. Sunlight means fluctuations in temperature, which can pop a cap or build up unwanted pressure inside the bottle.
Maintaining a cool, stable environment pays off. I worked at a facility where letting the temperature climb turned sealed bottles into pressure cookers. A fridge or low-humidity cabinet makes a massive difference. Keep your thermometer nearby and check it regularly—automation fails, and the nose rarely catches subtle changes until it’s too late.
Reject the idea of just storing this next to acids or bases. Even a slow leak can spark off fumes or cross-contaminate, setting off hazardous reactions. Flammable chemicals need dedicated, ventilated cabinets—metal ones with fire resistance, away from sources of sparks or static.
Closed spaces turn minor leaks into major headaches. My first encounter with thiophene-2-ethylamine happened in a room with poor airflow—a minor spill, and the place reeked for days. The solution was better air circulation. Fume hoods or filtered cabinets pull out vapors before they collect and start causing harm.
Routine checks clock small leaks early. Don’t trust seals forever: once every month or two, run your hand or a piece of pH paper around the cap and body to catch issues. Factory packaging lasts only so long before seals start to degrade.
The safest storage plan in the world doesn’t mean much if people aren’t following it. Update your teammates, run drills, and refresh signage. I’ve seen teams get complacent and—after months of no issues—slip up during a transfer or restocking run. Training turns safety from theory into habit.
A few crowd-sourced solutions make life easier—like group checklists, color-coded shelving, and alarms tied to humidity sensors. Switching lights to motion sensors near storage cabinets catches eyes and prevents accidental long stays in areas where vapors can build up fast.
Don’t let old bottles collect dust. Audit stock every six months; clear out what won’t be used soon. Never pour leftovers into sinks or regular trash. Specialized waste containers and pickup services exist for a reason; getting rid of leftovers responsibly keeps the environment—and colleagues—safe.
Thiophene-2-ethylamine brings together a five-membered ring of carbon and sulfur, with an amino group attached at the second carbon via a short ethyl chain. You get a molecule that’s shaped a lot like other amines you find in organic chemistry labs, but with a touch of sulfur that changes its physical qualities in the flask or bottle.
This organic chemical usually shows up as a colorless to pale yellow liquid. Its distinct, somewhat fishy or ammoniacal smell points to the nature of the amine group. If you’ve worked in synthesis labs, you probably recognize that aroma from similar small amines. It tends to stick around, even with the bottle closed tight. Once you open the cap, it announces itself fast.
Thiophene-2-ethylamine has a boiling point that sits a bit above water, falling in the 194-198°C range. Ambient pressure plays a role here, but most chemists do their distillation near atmospheric pressure. Melting point falls below room temperature, which leaves the chemical in liquid form under most lab and industrial conditions. Low melting points in chemicals like this can make handling straightforward at room temperature, but high temperature stability is important for people working in high-heat syntheses or reactions.
This compound dissolves well in common organic solvents, such as ethanol, acetone, and ether. It does not mix as easily with water due to the thiophene ring which is mostly nonpolar, though you still get limited solubility from the amine group. That versatility opens the door to use in various reaction schemes. As for density, it comes in slightly heavier than water, around 1.11 g/cm³ at room temperature. Pour it into a beaker of water and it sinks, which signals the presence of heavier atoms from sulfur.
Light and air can slowly change the appearance and properties of thiophene-2-ethylamine. If exposed for long enough, oxidation can yellow the liquid. This is a familiar story for many organic amines or sulfur-containing chemicals. Storing in amber glass, under nitrogen, or in the dark gives the best results if you want to keep it pure. Experience says old samples in a teaching lab will go off-color unless watched carefully, so rotation out of stock and checking sample clarity should be a routine habit.
Handling thiophene-2-ethylamine means paying attention to its volatility and strong odor. Good ventilation and gloves are essential. Its moderate boiling point means some evaporation can happen if left open. Splash or vapor exposure to the eyes or skin can cause irritation. The compound also carries flammability concerns, so keeping ignition sources away is a basic rule. I always store chemicals like this away from oxidizers, since the sulfur and amine both provide reaction sites for unwanted surprises.
Those who work with thiophene-2-ethylamine know its combination of physical properties—liquid at room temperature, heavier than water, organic solvent solubility, and ability to react through both its sulfur ring and amine group—all give it a real place in organic synthesis work. Reliable handling and good storage practices make sure it stays usable and safe through repeated projects. For anyone running regular syntheses or developing new thiophene-based compounds, the unique blend of physical qualities sets a stable foundation for further research.
| Names | |
| Preferred IUPAC name | 2-(2-Thienyl)ethan-1-amine |
| Other names |
2-Thienylethylamine 2-(2-Thienyl)ethylamine 2-Ethylaminothiophene |
| Pronunciation | /θaɪˈoʊfiːn tuː ˈɛθɪl.əˌmiːn/ |
| Identifiers | |
| CAS Number | [29443-49-8] |
| Beilstein Reference | 120924 |
| ChEBI | CHEBI:34618 |
| ChEMBL | CHEMBL317162 |
| ChemSpider | 130887 |
| DrugBank | DB08798 |
| ECHA InfoCard | 03e0651a-88b0-4f57-9704-2ef8657f162b |
| EC Number | 2683-77-8 |
| Gmelin Reference | 93094 |
| KEGG | C06373 |
| MeSH | D041751 |
| PubChem CID | 16657 |
| RTECS number | YN8220000 |
| UNII | 8U23Y7M16H |
| UN number | UN3431 |
| CompTox Dashboard (EPA) | DTXSID2021856 |
| Properties | |
| Chemical formula | C6H9NS |
| Molar mass | 111.18 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | ammonia-like |
| Density | 0.996 g/mL |
| Solubility in water | soluble |
| log P | 0.97 |
| Vapor pressure | 0.4 mmHg (at 25°C) |
| Acidity (pKa) | 9.56 |
| Basicity (pKb) | 7.09 |
| Magnetic susceptibility (χ) | -66.7×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.595 |
| Viscosity | 0.944 cP (20°C) |
| Dipole moment | 2.51 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 254.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -3938.8 kJ/mol |
| Pharmacology | |
| ATC code | N06BX11 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P273, P280, P302+P352, P305+P351+P338, P312, P332+P313, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 2-3-0 |
| Flash point | Flash point: 80 °C |
| Autoignition temperature | 320 °C |
| LD50 (median dose) | LD50 (median dose) of Thiophene-2-Ethylamine: "800 mg/kg (rat, oral) |
| NIOSH | NA |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.5 ppm |
| IDLH (Immediate danger) | NIOSH does not list an IDLH value for Thiophene-2-Ethylamine. |
| Related compounds | |
| Related compounds |
Thiophene Thiophene-2-carboxaldehyde Thiophene-2-acetic acid Thiophene-2-methanol 2-Bromothiophene 3-Thiopheneethanamine 2-Thiophenemethylamine |