Chemists uncovered many unique molecules during the early days of organic synthesis, and thiophene-2-carbaldehyde stands out as one product with real staying power. Its roots stretch back to the late 19th century when research into thiophene derivatives heated up alongside new developments in coal tar chemistry. Pioneers wanted building blocks with both aromatic and heterocyclic features, so they worked up ways to turn simple thiophenes into more complex aldehydes. By the mid-20th century, standardized preparation put thiophene-2-carbaldehyde in the hands of researchers worldwide. This allowed for a steady rise in both laboratory and industrial applications, with academic studies laying out its role in organic synthesis, medicinal chemistry, and materials research.
Thiophene-2-carbaldehyde, sometimes called 2-formylthiophene, delivers a sharp, distinctive scent and a yellow-brown hue. The molecule features a thiophene ring bonded to an aldehyde group at the two-position, which gives it reactivity for chemical modification. Commercial sources supply this compound at varying purity levels, mostly clear to slightly colored liquids packed in glass or plastic bottles. Suppliers indicate shelf life under cool, dry, and well-ventilated storage, out of sunlight to avoid decomposition.
The formula C5H4OS places it on the smaller end of organic intermediates. With a molecular weight just shy of 112 g/mol, thiophene-2-carbaldehyde carries volatility and a boiling point between 192–194°C. Its density measures slightly above water, and it dissolves moderately in common organic solvents but resists mixing with water. The compound releases a strong, sharp, somewhat nutty odor, common for many aldehydes, and darkens with age if left exposed to light or air. Its reactive carbonyl group opens doors for nucleophilic addition, condensation, and reduction, which downstream chemists depend on for diversification.
Product specifications cover a range of requirements: purity percentages (usually above 98%), refractive index, color, boiling point, and typical impurity profiles. Labels supplied with the product mention CAS number 98-03-3, hazard statements, and recommendations for safe handling. Industrial labeling must flag the potential for respiratory irritation and outline safe storage to meet both local and international transport standards. Quality documentation stretches past the basics—a robust certificate of analysis ensures each batch falls within required parameters for both research and manufacturing.
Industrial and lab-scale preparations lean on formylation of thiophene. Vilsmeier-Haack and Reimer-Tiemann reactions often provide reliable production routes. The Vilsmeier-Haack method typically treats thiophene with DMF and POCl3 under controlled temperature, giving high yields and easy work-up. Lab notebooks contain plenty of stories where small tweaks—different catalysts, temperature ramps—swing yields or impurity levels up or down. Scale-up brings its own challenges, such as the need for inert atmospheres and careful handling of byproducts, especially when sensitive functional groups show up in later reactions.
Altering thiophene-2-carbaldehyde’s structure keeps research chemists busy. It serves as a gateway for new heterocycles, pharmaceuticals, and polymer backbones. Treating it with various nucleophiles gives secondary alcohols or imines, while Wittig reactions convert the carbonyl into alkenes. Chemists often build multi-step syntheses around its versatility—building up from the aldehyde into alcohols, acids, hydrazones, or even complex cyclic products. Reductive amination adds amine groups, opening doors for bioactive derivatives, many of which show up in early drug discovery projects or as special ligands in coordination chemistry. Its role as a monomer or side chain in conductive polymers often begins with careful modification at the aldehyde group.
In addition to thiophene-2-carbaldehyde, chemists and suppliers know it under synonyms like 2-formylthiophene, 2-thiophenecarboxaldehyde, and α-thienylaldehyde. Chemical registries and supply catalogs keep things organized through numbers such as CAS 98-03-3 and EC 202-627-7. Some suppliers list it under regional naming conventions, but the structure and key identifiers provide clarity.
Safety officers and chemists pay close attention to handling guidelines. Thiophene-2-carbaldehyde can irritate skin, eyes, and the respiratory tract. Exposure controls in research settings rely on fume hoods, gloves, and splash-proof eyewear. Safety data sheets call for immediate washing if spills occur or if contact with skin happens. Fire risk from volatile organic fumes means rigid no-flame policies near storage and work areas. Emergency protocols often involve containment, ventilation, and waste handling via designated chemical waste routes. Regular training and updated safety protocols keep users informed about hazards, while international labeling provides consistency across borders.
Pharmaceutical research draws on thiophene-2-carbaldehyde’s versatility. Synthetic chemists value its functional group for forming intermediates on the road to new drugs, especially when sulfur heterocycles serve as bioisosteres. Materials scientists explore its applications in organic electronics and dyes, with the aldehyde facilitating further modification. Crop protection, fragrance synthesis, and specialty materials all benefit from the molecule’s ready reactivity. The flexibility to install or modify groups on the thiophene ring feeds creativity in both academic and industrial discovery labs.
R&D teams target both improvement in synthetic methodology and exploration of new applications. Laboratories report new catalysts and greener solvents to streamline its preparation, chipping away at waste and improving yields. Recent patents and papers showcase new derivatives, especially ones with enhanced biological or photophysical properties. Collaboration between academic groups and manufacturers has accelerated the search for new drugs or optoelectronic materials. Tweaks at the molecular level ripple outward, shaping everything from sensor devices to anticancer agents. Each small step forward in synthesis or reactivity improves access and opens uses previously out of reach.
Toxicologists examine exposure risks for both lab staff and end users. Acute inhalation or skin contact sometimes produces irritation, but there’s limited evidence of long-term or cumulative effects in small-scale laboratory work. Industrial operations add another layer of responsibility, prompting air quality monitoring and strict worker training. Animal studies report mild toxicity at high doses, pushing researchers to favor closed systems and personal protective gear. Regulatory bodies in Europe, the US, and Asia sometimes call for extra documentation before approving large-scale applications, particularly in food or pharma contexts.
Green chemistry initiatives and regulatory pressure on volatile organics spark substitutions and cleaner manufacturing, but the basic importance of thiophene-2-carbaldehyde likely keeps it relevant for years. Automated synthesis platforms and AI-driven drug discovery platforms both pull new value from such versatile compounds. Flight to safer or more sustainable solvents, catalyst recovery, and recycling efforts could make its preparation friendlier for the environment. On the application side, new molecular electronics, energy storage, and bioactive agent research continue to tap thiophene-2-carbaldehyde’s structure. Collaboration between suppliers, regulatory agencies, and end-users will shape updates in labeling, documentation, and safety practices, keeping the molecule in active circulation while reflecting both scientific and social priorities.
In the world of chemistry, few compounds stay under the radar like thiophene-2-carbaldehyde. I remember my early days in a research lab, sorting bottles of chemicals, unsure about the odd-smelling ones. You pick up a flask with this pale, slightly spicy scent, and realize it’s not just another solvent — it’s one of those rare building blocks that gets shuttled around in organic chemistry labs for a reason. Over the years, I’ve seen how it slips into all sorts of research and production cycles, quietly providing a cornerstone for discovery and innovation.
Drug researchers tend to rely on compounds offering both versatility and special chemical sites for tweaking structures. Thiophene-2-carbaldehyde stands out because its thiophene ring gives a punch of stability and electron richness. Its aldehyde group lets scientists attach all kinds of functional groups. Over two dozen research articles describe how it’s mixed into the making of new antivirals, antifungals, even parts of potential cancer medicines. These aren’t hypothetical uses — it plays a structural role, forming the backbone of molecules that target some of today’s tough diseases.
Chemists at flavor and fragrance companies count on molecules that offer depth and warmth, especially those hinting at earthy, nutty notes. My strolls past the “fragrance lab” at work always brought a rush of powerful aromas—some pleasant, some not. It’s not apple pie, but thiophene-2-carbaldehyde creates subtle hints in perfumes and food, especially when you want a roasted or bread-like undertone. It shows up in chocolatey and coffee additives, approved in tiny, managed quantities. The European Food Safety Authority lists it for use in foods, after careful safety testing.
Not everything ends up in a pill or perfume bottle. Chemical makers see thiophene-2-carbaldehyde as a starter for advanced polymers and dyes. The presence of both sulfur and the reactive aldehyde group means you can kick off reactions that stitch together long, useful chains. Some of the organic solar cell panels and light-emitting devices on the market contain materials that started their journey with this molecule. It may seem indirect, but small changes in starter chemicals can shift efficiency and durability of high-tech goods by several percent—a big deal at the industrial scale.
Any chemical packed with reactive sites deserves respect. A colleague once told me stories about accidental spills and the need for good airflow—the odor and mild irritation you get from thiophene-2-carbaldehyde remind you it’s a compound requiring careful handling. It doesn’t belong in uncontrolled environments. Regulatory groups classify it as a skin and eye irritant. Lab protocols stress eye protection, gloves, and fume hoods.
There’s no shortage of opinions about chemical use in everyday products. Some call for safer green chemistry alternatives. My own hope is that smarter design—using less toxic solvents and securing better waste capture—will let us harness molecules like thiophene-2-carbaldehyde for medicine, foods, and materials without risking health or the environment. Open-access data and regulatory oversight help. What matters most is staying curious and cautious, always weighing both innovation and responsibility.
Thiophene-2-carbaldehyde has the formula C5H4OS. Take a look at this structure and you’ll spot a five-membered ring—a telltale sign of the thiophene core. The sulfur atom gets locked in that ring, driving many of its chemical quirks. Place an aldehyde group directly next to that sulfur atom, and you’ve got thiophene-2-carbaldehyde.
In graduate school, I once spent a whole month in the lab just to run a single reaction with thiophene-2-carbaldehyde. The experiment showed me up close how such small organic molecules end up in everything from new drug candidates to specialty materials. Chemists value thiophene’s ring for its stable yet reactive properties. Adding the formyl group (that’s the aldehyde) on carbon 2 sets the stage for further modification. Students working with this compound learn fast that its smell lingers on your gloves long after you think you’re done.
This structure isn’t another arbitrary bit of trivia. Knowing where the functional groups land leads to smarter decisions about chemical design. In the lab, C5H4OS doesn’t just roll off the tongue—it guides every calculation, every prediction about how the molecule might behave. For instance, the electron-rich ring means overlapping orbitals, and the aldehyde tugs electrons in a way that changes its reactivity with common reagents. You can’t separate these facts if you want to build new materials or test for medicinal activity.
Students may groan about having to memorize chemical formulas, but the truth is, nobody designs new sensors, polymers, or pharmaceuticals without attention to these details. If you get the formula wrong, you risk the safety of your experiment or taint an analytical result. Having even a single atom out of place might throw off the solubility or the outcome of a synthesis.
Many young chemists, me included, learned this lesson after running into failed reactions that cost weeks of work. Safety officers stress these basics for good reason—accurate formulas underpin all record-keeping, safety data sheets, and process scale-up in manufacturing. Chemicals like thiophene-2-carbaldehyde often sit in the background, but their correct identification is critical for regulatory compliance, waste management, and inventory tracking. There’s more than academic interest riding on the right answer.
A strong scientific culture doesn’t just emphasize using the correct formulas in exams. Real improvement means double-checking labels, implementing barcode systems for inventory, and holding team members accountable. In the years since I first handled thiophene-2-carbaldehyde, our department started requiring chemists to not only label chemicals but also cross-check hazard information and formula entries every week. Mistakes dropped sharply—not because we became smarter, but because we made accuracy a habit.
For researchers, consistent use of the correct formula also smooths collaboration across borders. Teams on different continents working with thiophene-2-carbaldehyde use C5H4OS so the product quality stays the same from lab to lab. This shared language prevents costly errors, supports intellectual honesty, and protects those handling the substance.
Thiophene-2-carbaldehyde sounds like something from a graduate chemistry textbook. Truth is, it pops up in more places than many expect. Labs use it as a building block for pharmaceuticals and dyes. You can spot its chemical fingerprint in research settings. My college chemistry course once left a lasting impression after a careless sniff—this chemical’s sharp odor hits hard, and it’s no artificial grape.
Opening a bottle of thiophene-2-carbaldehyde releases a pungent, irritating vapor. The EPA scores it as having acute toxicity. Skin and eye contact spark burning and redness. Longer breathing exposure, especially in closed spaces, does more harm. Irritated airways, headaches, and nausea show up before anyone expects. In teenager-speak, “it’s not a good time.” Most of the trouble comes from people not giving it enough respect. One moment without gloves or goggles, a splash or fume exposure, and the lesson becomes crystal clear. Everyone in a lab should keep the Material Safety Data Sheet handy for reference.
The challenge with chemicals like thiophene-2-carbaldehyde is two-fold: workplace safety and the mystery of long-term risk. Short-term, spillages release irritating fumes and demand good ventilation. Chronic risk seems less certain. Right now, scientists haven’t tied this compound directly to cancer or birth defects, but “unknown” isn’t the same as “safe.” Small-scale hobbyists and even experienced techs sometimes let their guard down. I’ve seen a friend reach for a flask without gloves, only to learn the hard way why every container at the bench should carry a hazard symbol.
I recall fits of bravado in undergrad labs—bluffing through without masks or with rolled-up sleeves. After seeing rashes and headaches crop up after careless handling of organics, caution became second nature. Chemicals like thiophene-2-carbaldehyde draw a hard line between respect and regret. Folks working in the field can slip into complacency, especially when “I’ve done this a hundred times” starts to sound comforting. Stories pile up fast around lab benches and medical suppliers. One spill, one case of improper labeling, and suddenly, even trained staff end up in the emergency room.
The fix isn’t mysterious or out of reach. Good fume hoods, gloves, goggles, and clear labeling go a long way. Keeping proper storage away from heat or incompatible chemicals keeps surprise fires off the menu. Anyone handling this substance ought to make “no shortcuts” their motto. Emergency washing stations should always be within reach. Training sessions every six months serve as valuable reminders—I still attend, and there’s always a new trick or horror story to remember. Disposal matters too. Waste must go into marked, sturdy, sealed containers for safe pickup by professionals. Pouring leftovers down the drain or tossing them in the trash never ends well, for people or the environment.
Working with thiophene-2-carbaldehyde means facing the actual risks. With careful handling and respect for protocols, most problems become rare. Lax practices only turn minor mishaps into dangerous incidents. The chemistry world doesn’t leave much room for wishful thinking—good habits and respect for risk make the real difference.
If you’ve ever managed a chemical supply, you know that some compounds don’t forgive mistakes. Thiophene-2-carbaldehyde fits right in there. This liquid carries a pungent odor and can give you a headache if you get sloppy about handling it. That sharp, irritating smell hangs in the air if the cap isn’t tight. It stains skin and leaves yellowish marks on surfaces if there’s a spill.
Getting the basics right saves you from trouble. This chemical irritates the eyes, skin, and respiratory tract. Too much vapor in a poorly ventilated room causes coughing and watery eyes. If you’re not careful with open bottles on a work bench, the cleanup can turn into a real chore. Flammable vapors present another risk, making it a poor choice for cluttered shelves or old wooden cabinets near electrical gear.
Routine lab work taught me to keep anything that smells strong and evaporates quickly off the main workbench. Thiophene-2-carbaldehyde belongs in tightly sealed amber glass bottles. Light degrades it over time and the bottle color helps slow that down. Ventilated chemical cabinets cut down on vapor buildup, so a metal safety cabinet with an exhaust becomes the go-to spot for storage.
I have seen colleagues store such compounds with incompatible chemicals, but it leads to corrosion and even violent reactions over the years. This chemical reacts badly with strong oxidizers and acids. Mixing it even accidentally starts to corrode metal shelf brackets and stains paper labels for nearby bottles. A dedicated shelf for aldehydes or sulfur-containing reagents proves safest.
Temperature control matters here. Room temperature works, but avoid extremes. Heat speeds up decomposition and cold makes bottles more brittle, creating risks if you drop one. Always label bottles with the date and check for discoloration or crystal formation before each use. If you see cloudy liquid or sediment, don’t use it — make arrangements for safe disposal.
One habit I picked up from experienced lab staff involves using secondary containment. Stuff a leak-proof plastic tray under your storage bottles. Even a drop can stink up the room, but that tray catches spills before they reach the floor or mix with other chemicals. Spills can be contained and cleaned up using standard absorbents, then disposed of as hazardous waste.
Solid ventilation makes all the difference. Fume hoods aren’t overkill; they ensure vapor doesn’t reach unsafe levels, especially in smaller spaces. If you work late hours or alone, make sure someone knows what you are handling. Keep a spill kit with absorbent pads and proper gloves nearby. Eye wash stations and safety showers serve as important backup if something goes sideways.
The take-home lesson: Storing Thiophene-2-carbaldehyde safely saves time, money, and well-being. Treat it with respect, follow practical lab habits, and you’ll avoid chemical headaches — literally and otherwise.
Organic chemists don’t wander in the dark when preparing thiophene-2-carbaldehyde. The Vilsmeier–Haack reaction stands as an old friend in this work. Most chemists reach for this method because it fits well with thiophene’s electron-rich nature. In practice, the reaction means mixing thiophene with DMF and phosphoryl chloride. The mixture heats gently. The result: a formyl group lands right at the 2-position. For scale-up and reliability, few rivals have edged past this approach. Yields regularly reach 60–80%, so the process rarely leaves much to complain about—unless you’re hunting for perfect green credentials.
Pushing for greener chemistry isn’t just talk anymore. The Duff reaction gently nudges its way in for small-scale labs, especially where milder conditions earn extra points. Instead of strong halogenating agents, this approach uses hexamethylenetetramine with a bit of acid. Safety improves, though yields come down a notch. Fewer fumes, slightly muddy work-up, and sometimes higher costs. Still, I used this trick in grad school when the only hood was busy with something nastier.
Researchers have tried variations using ionic liquids and microwave assistance. The idea? Chop the reaction time, ditch some hazardous byproducts, or skip complicated separations. These remain useful for academic research but less so for bulk synthesis, where cost and practicality still win most arguments. Reports vary, but you find yields hovering near what you get from older techniques, if not slightly less. Labs focusing on sustainable practices keep their eyes on these newer versions, but industry hasn’t thrown its full weight behind them yet.
Beyond those familiar names, a Friedel–Crafts acylation can offer another avenue. Bringing together thiophene, an acyl chloride, and a strong Lewis acid such as AlCl3 lays the groundwork for further chemical finagling. This often means you have to tack on an extra step at the end: reducing the intermediate and then oxidizing it to finish with the aldehyde group in place. It’s a little longer, but if a lab already has these reagents, the route remains attractive. In practice, more steps mean more points of failure and extra purification—but sometimes you work with the tools at hand.
Making a few grams on a bench and running kilos through a plant raises very different concerns. For large-scale synthesis, the Vilsmeier–Haack route still pulls ahead. Operating teams know the risks: DMF and phosphoryl chloride come with toxicity and disposal problems. To manage these, companies invest in safety equipment, better waste treatment, and more training—but these add to the bottom line. During the early years in my career, we once spilled some POCl3 and learned the hard way why you move slowly and double-check connections. Accidents stick with you, and safety becomes more than paperwork.
What would improve the game? More investment in milder reagents and less wasteful procedures would help both smaller bottles in universities and tankers in chemical manufacturing. As interest in green synthesis grows, pressure mounts to swap out harmful solvents and cut down steps. The science is here—the question now leans on whether business, regulation, and demand move quickly enough to draw these into the mainstream.
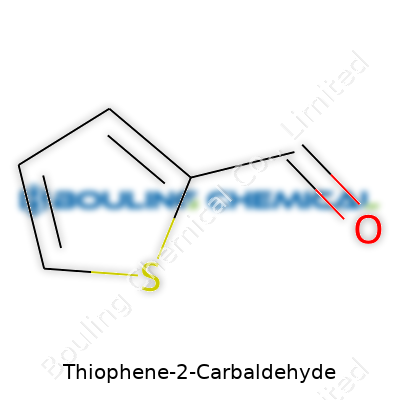
| Names | |
| Preferred IUPAC name | Thiophene-2-carbaldehyde |
| Other names |
2-Formylthiophene Thiophen-2-carboxaldehyde 2-Thienylaldehyde |
| Pronunciation | /θaɪˈoʊfiːn tuː kɑːrˈbæl.dɪ.haɪd/ |
| Identifiers | |
| CAS Number | 98-03-3 |
| Beilstein Reference | Beilstein Reference: 106055 |
| ChEBI | CHEBI:35418 |
| ChEMBL | CHEMBL60398 |
| ChemSpider | 13864022 |
| DrugBank | DB02170 |
| ECHA InfoCard | 100.011.057 |
| EC Number | 202-451-3 |
| Gmelin Reference | 80899 |
| KEGG | C06182 |
| MeSH | D013854 |
| PubChem CID | 6977 |
| RTECS number | YZ3150000 |
| UNII | I358086BWA |
| UN number | UN3432 |
| Properties | |
| Chemical formula | C5H4OS |
| Molar mass | 110.14 g/mol |
| Appearance | Colorless to yellow liquid |
| Odor | sweet; penetrating; strong |
| Density | 1.164 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 1.98 |
| Vapor pressure | 0.32 mmHg (25°C) |
| Acidity (pKa) | 7.0 |
| Basicity (pKb) | 13.36 |
| Magnetic susceptibility (χ) | -60.0e-6 cm³/mol |
| Refractive index (nD) | 1.570 |
| Viscosity | 1.181 cP (20°C) |
| Dipole moment | 2.95 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 179.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -15.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1875 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P210, P261, P280, P305+P351+P338, P312 |
| NFPA 704 (fire diamond) | 2-2-1-W |
| Flash point | 64 °C |
| Autoignition temperature | 175 °C |
| Explosive limits | Explosive limits: 1.2-8.4% (in air) |
| Lethal dose or concentration | LD50 (oral, rat): 1800 mg/kg |
| LD50 (median dose) | LD50 (median dose): 500 mg/kg (rat, oral) |
| NIOSH | NV1750000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Thiophene-2-Carbaldehyde: Not established |
| REL (Recommended) | 0.5 ppm |
| Related compounds | |
| Related compounds |
Thiophene Thiophene-2-carboxylic acid Thiophene-3-carbaldehyde Furfural Benzaldehyde |