Chemists have long explored thiophene derivatives for their unique structure and reactivity. By the early 20th century, researchers recognized the potential of compounds with both aromatic sulfur rings and alcohol groups. Laboratory synthesis of Thiophen-2-ethanol began to gain traction as scientists searched for intermediates capable of bridging organic synthesis and pharmaceutical development. Patents and journal articles from the 1950s onward often traced their steps through sulfur-containing heterocycles. Through both targeted research and broader studies aimed at building block molecules, this compound emerged as a bridge linking sulfur chemistry with alcohol functionality—enabling new research into synthesis and potential uses in medicine, flavor, and advanced materials.
Thiophen-2-ethanol offers a combination of a thiophene ring and a primary alcohol decoration at the two position. Its molecular formula, C6H8OS, and molar mass close to 128.19 g/mol, keep it light enough to handle easily and reactive enough for modifications. Its faint, slightly unpleasant odor and pale-yellow to colorless appearance reflect its use as a specialty building block. Producers design packaging with chemical-resistant liners and high-integrity seals. Researchers and companies from pharmaceuticals to petrochemicals seek it for its blend of chemical utility and manageable handling.
At room temperature, Thiophen-2-ethanol appears as an oily liquid with moderate volatility. It blends smoothly in many organic solvents like ether, chloroform, and DMSO. With a boiling point just above 220°C and melting point near -30°C, it resists freezing in standard labs and most warehouses. The density hovers close to 1.15 g/cm³. The hydroxyl group at the ethyl tail serves as the launchpad for esterification, ether formation, and oxidation, while the aromatic ring brings in strong resonance stabilization and sulfur’s electron-donating effects. This dual identity gives it flexibility for functional group transformations and new molecule assembly.
Chemical suppliers ship Thiophen-2-ethanol at purities usually no less than 97%, sometimes refined for research demands with 99% minimum. Labels clearly display the CAS number (usually 5402-55-1) and UN hazard codes if it rides as a liquid cargo. MSDS sheets call out both chemical and physical characteristics, recommended storage at 2–8°C away from light, and incompatibility with strong oxidizers. Certificates of Analysis back up assays for alcohol content, water percentage, and trace sulfur byproducts. Shipment records list regulatory compliance for REACH in Europe and TSCA in the United States, helpful for cross-border manufacturing.
Synthetic routes to Thiophen-2-ethanol track back to simple building blocks. A common pathway runs through thiophen-2-carboxaldehyde via reduction, often using sodium borohydride or catalytic hydrogenation. Some labs turn to Grignard reactions, coupling thiophene derivatives with ethylene oxide or related intermediates. Batch sizes range from a flask in pharmaceutical research to hundreds of kilograms in aroma and specialty chemical manufacture. Purification steps may include vacuum distillation and liquid-liquid extraction to remove colored impurities and sulfur-containing side products. Operators need both chemical know-how and process discipline.
The alcohol functionality attracts many modifications. Researchers oxidize it to thiophen-2-carboxaldehyde or thiophene-2-acetic acid as intermediates for even more complex synthesis. Tosylation, acylation, and ester formation broaden the spectrum. Electrophilic aromatic substitution on the thiophene ring allows for halogenation or nitration, setting the stage for advanced medicinal chemistry. In polymer research, the ethanol group serves as the anchoring site for chaining or cross-linking, improving flexibility within conductive or optical materials. The dual reactivity supports both academia and industrial product development.
Chemists and catalogues know this molecule by multiple names: 2-(Thiophen-2-yl)ethanol, 2-Thienylethanol, β-Thienylethanol. Distributors sometimes abbreviate or reorder descriptors; the most common commercial names mimic the IUPAC organization for clarity. Product codes reflect both supplier internal tracking and compliance with global trade requirements. Accurate labeling and documentation support inventory management for bridge compounds in research, scale-up, and downstream processing.
Handling guidelines owe their detail to both chemical properties and regulatory oversight. Users should avoid prolonged inhalation and skin contact, even though the acute toxicity ranks as moderate. Operators wear gloves of nitrile or butyl rubber, safety goggles, and splash-resistant coats. Ventilated hoods minimize airborne concentrations. Storage containers include secondary containment for liquid spills, and companies ensure waste disposal falls in line with local rules for organosulfur compounds. Emergency procedures emphasize quick response for spills and readiness with class B fire extinguishers, reflecting its flammability profile. Documentation, labeling, and internal audits anchor plant safety.
Thiophen-2-ethanol moves through a range of application niches. In flavors and fragrances, it can build sophisticated natural aromas, adding subtle earthiness and complexity when blended or reacted into larger molecules. Medicinal chemistry values its capacity to anchor drug candidates, particularly where sulfur’s electronic profile plays a functional role in biological activity. In material science, the molecule enables synthesis of new conductive polymers, often finding its way into research around organic electronics and flexible circuits. Laboratory research, in both academic and industrial development, treats it as a versatile intermediate for rapid assembly of high-value products.
Scientists continue probing the full capability of thiophene alcohols. With ongoing expansion in green chemistry, researchers design new, milder reduction routes that cut down on hazardous reagents and lower energy demand. Biotechnologists run screening studies for enzyme-catalyzed modifications, mapping new ways to tailor both the alcohol and aromatic domains. Pharmaceutical innovators test analogues in cell and animal models, balancing the promise of heterocyclic drugs with challenges around bioavailability and metabolic stability. Industrial cooperation helps drive continuous improvement, whether optimizing yields in batch reactors or pioneering new product categories blending electronics with drug delivery.
Careful examination tracks both acute and chronic exposure. Animal studies identify LD50 values that flag moderate toxicity through oral and dermal routes. At low concentrations, skin and eye irritation may develop with repeated exposure, prompting clear workplace controls. In the environment, researchers look for breakdown products and bioaccumulation patterns, so agencies can draft informed regulations. Longer-term data explore organ-specific impacts and the potential for mutagenicity. Transparent reporting and systematic lab testing shape both workplace policies and product stewardship.
Momentum behind sulfur-containing intermediates grows as new technologies call for special performance from low-weight, heteroatom-rich building blocks. Future research will emphasize green production, looking to biocatalysis and solvent-free approaches. Rapid screening under computational models may reveal new pharmaceutical leads, especially as interest in modulators of oxidative stress intensifies. In organic electronics, improved hybrid materials that combine thiophene’s charge-carrier qualities with the processability of alcohols create opportunities in next-generation sensors and flexible displays. Regulatory harmonization and enhanced safety profiling will support global trade and expand the toolkit for synthetic and application chemists. Every advance brings complications, so ongoing research into exposure and lifecycle will shape sustainable practices in labs and factories.
Most people walk through life without hearing about thiophen-2-ethanol. It’s not something your neighbor brings up at a barbecue. Yet this small compound with a mouthful of a name pops up in more corners of modern life than many realize. I remember catching its distinct aroma the first time during an internship at a fragrance lab. The experience stuck with me—a chemical that says so little in textbooks, but works quietly in so many industries.
Thiophen-2-ethanol carries a light, somewhat earthy smell, sometimes reminiscent of roasted grains. In the world of perfumery, that’s a gold mine. Perfume creators rely on it to bring subtle warmth and complexity to blends. Rather than making a fragrance loud and punchy, it anchors sweeter or floral notes, helping them feel rounder and more grounded. Many "niche" perfumes on boutique shelves draw on this molecule without making it the star of the show. It works like a background instrument—important, but hardly noticed when played just right.
The magic doesn’t stop with perfume. Food scientists also reach for thiophen-2-ethanol. It shows up in the creation of flavors for processed foods, especially baked goods and chocolate. In these foods, a little goes a long way—too much, and the taste can go off-kilter. But with the right touch, it deepens flavors, making them last longer and feel more genuine. Research from the Journal of Agricultural and Food Chemistry describes its presence in roasted coffee and even in some types of whiskey, where it comes as a natural byproduct of fermentation.
Pharmaceutical labs have also found value in this molecule. Chemists use thiophen-2-ethanol to build more complex compounds—think of it as one block in the construction of new drugs. Its particular structure allows it to act as a stepping-stone, introducing flexibility in synthesizing therapeutic agents, including some antivirals and central nervous system drugs. Without reliable access to such intermediate chemicals, development would stall and costs would climb. The push toward “green chemistry” has sparked more sustainable methods of making thiophen-2-ethanol, reducing waste and supporting safer workplaces.
No talk of chemicals can ignore safety. A compound that can enhance chocolate or perfume can still be hazardous in raw form. Handling it means proper training and ventilation, gloves, and respect for regulatory guidelines. The European Food Safety Authority and U.S. Food & Drug Administration both keep thiophen-2-ethanol on a tight leash, requiring disclosure when added to products. Transparency matters, especially as consumers demand clearer labeling and accountability from food and fragrance companies.
Seeing industry move away from petrochemical origins to plant-based synthesis offers hope. Biotech firms are engineering yeast strains to produce it more cleanly. This shift not only supports environmental goals, but also responds to a growing demand for “natural” ingredients. Supporting continued research and better labeling gives people more choice without losing out on safety or innovation. Science can build trust by inviting conversation about what goes into the things we eat, smell, and touch every day.
Thiophen-2-ethanol is not just another molecule with a tongue-twister name. Think about a simple ethanol backbone, two carbon atoms and a hydroxyl group, but then twist it a bit. The real character comes from a five-membered aromatic ring, thiophene, which replaces the usual benzene in familiar alcohols. This thiophene ring, centered around a sulfur atom and four carbons, grabs attention for its chemical and biological behavior.
Let’s lay out the structure. The core consists of a thiophene ring, a small circle of four carbons and a sulfur atom. At the position right next to the sulfur (known as the 2-position), a two-carbon chain dangles off the ring, ending in an alcohol group (–CH2CH2OH). Chemists sketch it as C6H8OS. The layout gives this molecule its physical and interactive quirks.
Working with aromatic compounds brings a lot of surprises. Thiophene, because of its sulfur, changes how these molecules participate in reactions. This weird little ring boosts stability, resists some types of chemical breakdown, and carries a distinctive smell—sometimes earthy, sometimes sweet. Adding that ethanol part at the 2-position gives it more flexibility, blending properties of aromatic and aliphatic compounds. You spot these ideas all the time in labs, especially when tailoring new syntheses or designing fragrances and pharmaceuticals.
From personal experience, putting together compounds like thiophen-2-ethanol often changes the game in organic chemistry syntheses. Pure ethanol groups stick to their usual roles, pairing up for hydrogen bonding, affecting how molecules dissolve, or even how they taste and smell. Thiophene’s aromatic flair offers unique handles for further modifications; swap groups, try coupling or create new heterocycles. I’ve mixed these in the flask and watched as properties in solvents or reactions shift, just from swapping the ring or moving a side-chain.
Plenty of people never see the side of science that relies on these subtle structural tweaks. For example, the sulfur atom in thiophene can interact with metals during catalysis or stick to biological targets other simple rings miss. This opens doors for researchers working on biochemistry or drug discovery. Some derivatives act as fragrance enhancers or build blocks for drugs—petite steps from the structure towards function.
In practical terms, safe handling and toolkits matter. I have run into lab hazards from thiophene derivatives being volatile or reactive under the wrong conditions. Storing pure samples, using well-sealed containers, and maintaining good ventilation proved vital. Lab work with thiophen-2-ethanol reveals how odor, flammability, or skin contact issues become factors. These direct experiences shape my trust in basic protocols and push for safer, greener chemical processes.
Building on foundational structures like thiophen-2-ethanol feeds into wider goals. Greener synthesis routes mean selecting methods that cut waste, shrink energy use, and steer away from toxic reagents. By designing better catalysts that respect this molecule’s quirks, chemists can keep science productive while watching the environment. Open access to data, safer lab practices, and wider education in aromatic chemistry can raise the bar collectively. Not every molecule will become a blockbuster, but understanding each one at this level makes progress across healthcare, technology, and even the subtle art of perfumery.
At first glance, chemical names spark a lot of worry. Thiophen-2-Ethanol doesn’t sound like something you want in your coffee. Its place in the world often links to labs and manufacturing instead of the kitchen table. For people who work around chemicals, understanding the safety of each bottle means more than scanning the label—it’s about trusting what the science tells us, and what real safety data shows.
This substance carries a bit of an odd smell, thanks to its roots in sulfur chemistry. It pops up in fragrance labs and sometimes in flavor work. The real question sticking to it, though, is whether it brings genuine risk—either to workers who handle it or folks further down the line.
Hazard data paints a careful picture. On material safety data sheets, you’ll often see warnings about eye and skin irritation. Breathing in a lot of vapors could cause dizziness or sore throat. Spilling it on your hands may lead to redness if you don’t wash up soon. Perhaps that sounds alarming at first, but think about how many household cleaners show the same set of warnings. Irritation doesn’t jump straight to “deadly poisoning.”
I’ve talked with chemists who spend years among glassware and gloves. They treat every unknown bottle with respect, but they know not every warning label means disaster if you play by the rules. Published studies do not show this compound as cancer-causing or linked to huge long-term health risks at normal exposure levels. The biggest risk sits with not using gloves, not running a fan, or ignoring spilled material. Skin exposure and accidental splashes cause most lab headaches—not subtle, life-altering harm.
The U.S. Environmental Protection Agency and European regulators both weigh in with similar takes: irritation matters, yes, but acute toxicity (the amount where real damage sets in) stands quite a bit higher than everyday workplace contact. So, workers with proper ventilation and gloves will not face life-changing risks from a splash or a little mist in the air.
Trust starts in honest discussion, especially where chemicals and daily work cross paths. Exaggerating hazards drives people away from science. Downplaying them hurts workers who already might not have a voice. Thiophen-2-Ethanol fits in this middle ground. It needs respect, not panic. Gloves, goggles, a careful cleanup routine, and a bit of training do most of the heavy lifting here.
OSHA and workplace safety groups boil things down to clear steps: limit skin contact, keep spills contained, make sure the room doesn’t fill with fumes. This works because most emergencies start small—a splash, a cloud, a forgotten leaky cap.
My experience in lab safety taught me some truths that hold up, no matter the chemical: don’t let worry run the show, and don’t let carelessness walk through the door. If working with thiophen-2-ethanol, use what protects you—proper gloves, working eye-wash stations, clear spill response plans. Talk openly about exposure risks, and make sure everyone knows what to do if a mistake happens.
No chemical becomes truly safe or toxic on its own. Context, habits, and honesty make up the real story. With this substance, the risk comes down to how you handle it—not whether it sits on a shelf at work or makes headlines online.
Years of work in university labs and small industrial settings drilled a habit into me: treat each bottle as unique, and don’t cut corners. Thiophen-2-Ethanol stands out as a reminder of how simple habits save headaches. Unlike table salt or vinegar, even low-exposure chemicals can go wrong fast without the right handling. Thiophen-2-Ethanol won’t explode if you look at it funny, but leaving a cap off or tucking it near a hot radiator leads to ruined material, lab cleanup, or even a risk to your health.
I once opened a cabinet to the aftermath of a cracked bottle. The building’s old heating system had spiked, and chemical fumes filled the air. Storage isn’t about convenience. Thiophen-2-Ethanol, being a liquid with a moderate vapor pressure and a tendency to degrade in contact with air, calls for air-tight containers, usually amber glass. Not all plastics block vapor reliably, and a loose or corroded cap invites both evaporation and impurities. Sticking with well-sealed glass, stashed upright and away from reach, became the unbreakable rule in our group after that day.
Leaving bottles near a sunny window or on top of a warm fridge pushes up temperature swings and helps break down organics. Many treat “room temperature” as a wildcard, but in practice, it means a spot between sixteen and twenty-five degrees Celsius, shielded from heat sources. Too cold or too hot risks crystal formation or degraded product. Once, a colleague lost an entire lot because the storeroom ran above thirty during summer. Checking for stable coolness matters. Sometimes, a dedicated flammable/chemical storage fridge saves both money and safety headaches over time, as insurance from chemical spoilage pays for itself.
Thiophen-2-Ethanol can pick up water if kept in humid, open environments. Even trace water triggers changes—sometimes cloudiness, sometimes shifts in chemical reactions. Simple tweaks, like tossing in a silica gel pack and double-checking the cap, hold contamination at bay. Reclosing after every pour and never dipping used pipettes back in stops mistakes. It is much easier to practice care upfront than troubleshoot weird results in the lab later.
Accurate labels mean more than avoiding confusion. Every time a bottle gets relabeled with a fresh date—batch, name, hazard symbols—anyone who walks in knows what they face. Locking cabinets, using spill trays, and tracking use in a chemical logbook reflect responsible handling. I once saw a shelf marked only with “reagents”; nobody wanted to be the first to touch them, and costly disposal followed. Good records mean less waste and safer rooms for everyone.
Training goes beyond reading a label. Every staff member should know the drills: how to spot leaks, wear gloves, run the fume hood, and deal with waste. A culture built on respect for chemicals keeps surprises rare. In labs I trusted, new faces always shadowed a veteran for the first few weeks. This habit passed down best practices that no manual fully covers and highlighted decisions about which materials should never sit side by side.
Storing chemicals like Thiophen-2-Ethanol well isn’t about overkill. It’s about heading off issues before they land. That level of care shows respect for both your work and the people around you, and minimizes risks that never need to become stories in the breakroom.
Thiophen-2-ethanol brings a mix of curiosity and precision into any chemistry discussion. Pressed through the lens of organic synthesis, its molecular weight isn’t just a number on a spec sheet. For reference, the molecular weight of Thiophen-2-ethanol stands at 128.19 g/mol, and this figure tells more than just mass. It steps into every calculation whether you're preparing reagents, prepping standards, or crafting pharma intermediates. For folks working in labs, getting a single digit wrong twists up entire experiments. I’ve seen it happen—a couple milligrams off can force a researcher back to the very start.
Look at the formula: C6H8OS. Count up the pieces—six carbons bring 12.01 each, hydrogen adds its light weight at 1.01 for eight atoms, oxygen weighs in at 16.00, and sulfur brings the heft at 32.07 g/mol. Any chemist who’s set foot in a lab knows to double-check because these numbers shape batch sizes and reaction outcomes. Roundoff errors go beyond just the math; they creep into product purity and research reliability.
We live in a world where new drugs or materials can roll out thanks to getting these details right. Let’s say you’re scaling up from bench to pilot plant. Small deviations in the molecular weight calculation can shift yields by grams or even kilos. That’s a lesson I learned the hard way after a failed catalysis project at graduate school—the project manager turned bright red after discovering we’d been off by fractions of a gram at every stage. Multiply that by thousands and you see where small numbers spiral.
Safety officers always drill home the importance of accuracy, and molecular weight calculation anchors their warnings. Thiophen-2-ethanol, like many organosulfur compounds, brings unique challenges in handling and waste management. Underestimating required materials isn’t just a productivity issue—it can mean the difference between a safe experiment and a risky one.
Pharma labs, chemical plants, startups—they run on trust and audit trails. Quality control depends on properly identified raw materials. Any deviation in molecular weight figures opens the door to regulatory nightmares or product recalls. With chemicals like thiophen-2-ethanol, supply chains bank on the right numbers so certificates of analysis line up, and auditors stay happy. Most professionals who’ve seen recalls know that paperwork counts as much as chemical knowledge.
There’s still room to make life easier for both researchers and manufacturers. Open-access chemical databases grow every year, promising more sources for accurate values. Encouraging scientists to double-check numbers—from digital sources to textbook standards—makes experiments more robust. Lab software increasingly flags inconsistencies, alerting users before costly mistakes happen. As someone who’s been there, I vouch for an extra minute spent calibrating a scale or checking a molecular weight in a trusted database.
Reliable chemistry relies on details. Getting the molecular weight of thiophen-2-ethanol spot-on shapes every stage, from weighing out a starting material to signing off on a final report. Earning trust in science or industry takes precision, and it starts with numbers as basic as molecular weight. Every student, lab tech, or QC specialist benefits from the reminder that careful calculation fuels every breakthrough or routine result.
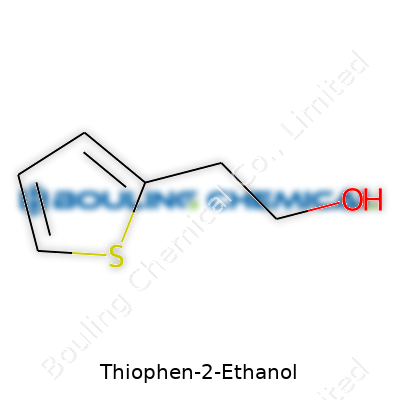
| Names | |
| Preferred IUPAC name | 2-(Thiophen-2-yl)ethan-1-ol |
| Other names |
2-Thiophenemethanol 2-(Hydroxymethyl)thiophene Thiophene-2-ylmethanol Thiophen-2-ylcarbinol 2-Thienylmethanol |
| Pronunciation | /ˌθaɪ.oʊˈfɛn tuː ˈɛθ.ə.nɒl/ |
| Identifiers | |
| CAS Number | [Fact: 'Thiophen-2-Ethanol' CAS Number is 5402-55-1] "5402-55-1 |
| Beilstein Reference | 1208731 |
| ChEBI | CHEBI:34582 |
| ChEMBL | CHEMBL509686 |
| ChemSpider | 53113 |
| DrugBank | DB08797 |
| ECHA InfoCard | DTXSID7065163 |
| EC Number | 202-246-2 |
| Gmelin Reference | 126454 |
| KEGG | C06381 |
| MeSH | D013860 |
| PubChem CID | 73076 |
| RTECS number | XN8575000 |
| UNII | K5PV12DX9F |
| UN number | UN3439 |
| CompTox Dashboard (EPA) | DTXSID7020632 |
| Properties | |
| Chemical formula | C6H8OS |
| Molar mass | 140.19 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | sweet, earthy |
| Density | 1.129 g/mL at 25 °C(lit.) |
| Solubility in water | Slightly soluble |
| log P | 1.64 |
| Vapor pressure | 0.0152 mmHg (25°C) |
| Acidity (pKa) | 14.3 |
| Basicity (pKb) | 13.86 |
| Magnetic susceptibility (χ) | -60.94·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.5790 |
| Viscosity | 1.54 mPa·s (25°C) |
| Dipole moment | 1.81 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 354.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –34.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3810.6 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | Precautionary statements: P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 104 °C |
| Autoignition temperature | 255 °C |
| Lethal dose or concentration | LD50 (oral, rat): 2,670 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 820 mg/kg |
| NIOSH | TTZ6300000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.5 - 1.0 mg/L |
| Related compounds | |
| Related compounds |
2-Thiophenemethanol Thiophen-2-ylacetic acid 2-Bromoethylthiophene 2-Ethylthiophene Thiophen-2-ylmethylamine |