Thiomorpholine’s story traces back to the early 20th century, when chemists dug deeper into heterocyclic compounds. Back then, the curiosity around molecules with both nitrogen and sulfur in a single six-membered ring pushed many to experiment with related structures. By the 1930s and 1940s, as labs expanded their capabilities, thiomorpholine earned attention for its unique chemistry compared to the more familiar morpholine. As synthetic chemistry took off in the postwar era, researchers grew increasingly interested in compounds that balanced reactivity and stability. The presence of both sulfur and nitrogen turned out to be a productive playground for people hunting for new pharmaceuticals, rubber additives, and specialty solvents. Every milestone in organic synthesis—new routes, better yields, cleaner reactions—fed the growth of thiomorpholine’s production and use.
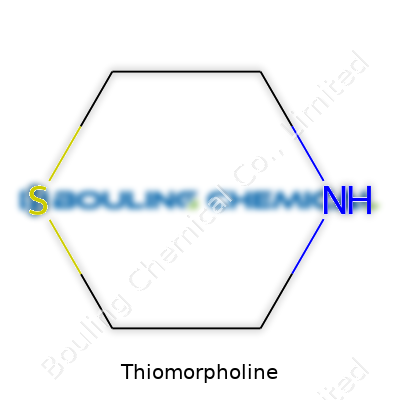
Thiomorpholine sits among a small handful of six-membered heterocycles, featuring a structure with one sulfur and one nitrogen occupying opposite corners in its ring. Its formula—C4H9NS—makes it a cousin to morpholine, but the sulfur atom shakes up its reactivity and potential uses. You will find thiomorpholine in the form of a clear or faintly yellowish liquid. It’s got a recognizable, sharp odor, not all that pleasant, probably retracing to its sulfur component. On paper, it almost seems simple, but the chemical’s balance of nucleophilicity and basicity owes a lot to the way both N and S bond together in the backbone.
At room temperature, thiomorpholine comes as a liquid with a boiling point around 169-171°C and a melting point near -28°C. Its density stays close to 1.13 g/cm³, which tells you it’s a bit heavier than water. Solubility plays a big part in its utility; thiomorpholine mixes easily with water and most organic solvents, lending flexibility to chemists looking for versatile building blocks. As a weak base, it accepts protons—enabling wide-ranging uses in synthesis and as a feedstock. Exposure to air leads to slow oxidation, which eventually forms sulfoxides or sulfones—this can become a problem in storage if no one pays attention. Flammable vapors create a safety focus, especially for warehouses storing large drums of the stuff. Mixing with strong acids or oxidizers always needs respect—both sides can ignite or break down the compound.
Suppliers label pails or bottles of thiomorpholine with the UN number 2810, signaling toxic liquid of organic type. Companies use CAS Number 123-90-0. Safety phrases often include warnings about skin, eye, and respiratory irritancy. Analysts want to see purity above 98%, moisture below 0.5%, and little to no visible impurities. On the shipping paperwork, you will spot hazard pictograms for health risk and flame, with proper packaging demanded for both land and air transport. Testing for trace amines and S-oxides helps weed out old or degraded material. Barcode tracking and digital batch records have become norms for supply chain transparency.
Thiomorpholine’s industrial synthesis starts with diethanolamine or morpholine, moving via chlorination or other activation, so nucleophilic substitution brings in a thio group. A typical route passes through diethylene glycol, which reacts in steps with hydrogen sulfide and ammonia. Every chemist who wrestles with this process learns to handle hydrogen sulfide’s toxic and stinky temperament. Clever catalyst systems, dry conditions, and steady temperatures help keep unwanted byproducts from building up. Over the years, researchers kept polishing these methods to bump up yield and purity, with continuous flow reactors offering more control for modern plants. Waste streams need attention, since sulfur waste and amine salts can both cause environmental headaches. Recovery units and closed-loop processes increasingly stand as industry standards for sustainable production.
Thiomorpholine opens up a toolkit of synthetic options. Its nitrogen provides an anchor for acylation, alkylation, and N-oxidation. On the sulfur side, chemists take advantage of S-alkylation, S-oxidation to sulfoxides (which often behave totally differently in reactions), and further oxidation to sulfones—especially useful when making bioactive molecules. Ring-opening reactions under strong acid or base conditions produce linear products used further downstream. Reaction with isocyanates or halides generates thiomorpholine derivatives with new properties. Pharmaceutical research often hangs on subtle tweaks made through selective functionalization on either the N or S atom, as this shifts both pharmacokinetics and toxicity. The possibilities stretch a long way, and each lab or factory seems to find a favorite method for the task at hand.
You’ll run into thiomorpholine under a handful of alternate names. The trade and lab catalogs list it as 1,4-thiazane, tetrahydro-1,4-thiazine, and thiamorpholine. People in pharma development sometimes call it tetrahydrothiazine. Packages carrying GHS labels could refer to it as morpholinesulfur analog. Synonyms pop up mostly because naming conventions split by field—what makes sense to an organic chemist might confuse a chemical engineer until both see the raw structure.
Working with thiomorpholine challenges anyone not used to sharp odors and toxic vapors. The compound poses acute risks to skin, eyes, and mucous membranes. Even a small spill means you need gloves, goggles, and good ventilation. Industrial settings bring in fume hoods, spark-proof equipment, and double containment for storage tanks. The liquid burns easily with an unsteady flame, so strict no-smoking policies and explosion-proof switches cut down fire risk. SOPs lay out containment, neutralization, and spill response plans, demanding rehearsed drills. Waste handling places a focus on chemical resistant containers and proper pH adjustment before drains. Annual medical checks are not uncommon in places where workers come close to large volumes. Transport by road or rail follows ADR or IMDG code, making sure containers resist impacts and stoppers never leak.
Industries love thiomorpholine for the same qualities that make it interesting to chemists. Rubber makers blend it into accelerators and anti-degradants that add years to tires, hoses, or industrial belts. Water treatment outfits favor its derivatives as corrosion inhibitors, especially for pipelines exposed to tough weather or chemical loads. Pharmaceutical labs see thiomorpholine as both a target and a stepping-stone: new drugs—particularly those fighting bacterial infection or controlling the CNS—often launch from thio-derivatives. Agrochemical companies investigate it for both herbicide and fungicide candidates. The solvent field looks for thiomorpholine-based blends that handle tough or sulfur-rich contaminants. Even electroplating circles have poked at its possibilities, as its S and N atoms coordinate metals in unusual ways. Most sectors stick to small volumes, but the rubber and water treatment worlds sometimes place ton-sized orders.
Labs keep chasing ways to pull more value from thiomorpholine’s scaffold. Medicinal chemistry now aims to build selective kinase inhibitors or enzyme blockers leveraging its heterocyclic core. Materials science groups believe its derivatives could improve flame resistance or elasticity in specialty polymers. Analytical research has sharpened sensitivity for thiomorpholine detection in workplace air and water streams, using HPLC, mass spectroscopy, or spectrofluorometry. Green chemists tinker with biosynthesis or bio-based feedstocks, hoping to bypass traditional hazards in production. Stable isotope labeling—using thiomorpholine as a tag—helps in tracking metabolic pathways in both pharmaceuticals and agricultural science. The collaborative push between universities and private industry continues, with more patents and cross-disciplinary publications every year.
Toxicologists put thiomorpholine through the wringer. Acute exposure studies show harm to skin, lungs, and eyes, matching the experience of any chemist who ever caught a splash in the lab. Animal studies paint a picture of moderate oral and inhalation toxicity, with LD50 values around 500-800 mg/kg (oral, rat). Chronic exposure data is thinner, but current studies suggest the compound does not pile up in fatty tissues—less long-term risk, though repeated exposure causes sensitization or dermatitis in some lab workers. Water solubility means spills risk rapid movement in drainwater, requiring real management at disposal. Testing in aquatic systems reflects moderate risk for fish and invertebrates, which feeds into local regulations wherever manufacturers operate. With every few years, agencies revisit the safety standards, nudged on by new studies and sometimes by pressure from local environmental groups.
Thiomorpholine’s place looks steady for now, with a solid base in rubber and water treatment. As pharmaceutical pipelines widen, more designers pick heterocyclic templates—this almost guarantees continued niche demand as labs hunt for novel synthesis routes. Greener production methods will probably pick up, nudged by government pressure and basic economics. Research could open up uses in electronics, as components in flexible displays or battery additives. Without breakthroughs, bulk growth might be slow, but specialty chemicals often enjoy long lives near the edges of the mainstream. Strong safety records and tighter regulations will shape handling and transport, especially in regions tightening toxic storage laws. Watching how universities and industry co-develop applications will tell much about the shelf life and versatility thiomorpholine brings to tomorrow’s toolkit.
Thiomorpholine sounds complicated, but break it down and you’re looking at a six-membered ring made from four carbons, one nitrogen, and a sulfur atom. Chemists love rings like this. You find them at the center of all sorts of reactions, mostly because this little molecule packs versatility into a small package.
I learned about thiomorpholine in a university lab, sweating over an annoyingly sensitive reaction that needed a tough, basic nitrogen sitting close to a soft sulfur. That’s basically the personality of thiomorpholine. It’s most famous for being a handy starting material in pharmaceutical labs. Picture it as a springboard — drugs for blood pressure, viral infections, and even certain antibiotics have sprouted from it. Merck’s blockbuster treatment for Hepatitis C, for example, draws from a molecule containing a thiomorpholine skeleton. This isn’t just trivia for chemistry exams. Companies invest real money in these molecules, patenting subtle twists on the skeleton in hopes of tweaking biological activity.
Outside of pharma, thiomorpholine also steps into the role of a chemical intermediate. At a plant I once visited, engineers used it for synthesizing specialized solvents. These solvents show up in oil refineries and gas processing where they help snatch sour-smelling hydrogen sulfide or carbon dioxide out of the air. Thiomorpholine, with that combination of nitrogen and sulfur, binds unwanted gas byproducts tightly enough to clean up the workspace but releases them without much fuss when conditions change. That means you save money and materials. In some specialized cases, these solvents outperform older amines by absorbing more gas, more quickly, and with fewer nasty corrosion side-effects.
Materials scientists also put thiomorpholine on their lists. Turn it into larger polymers, and you get coatings and adhesives that resist weathering and chemical attack. These materials can line industrial equipment or glue together composite panels that shrug off moisture and heat better than standard glues. Based on my experience working with adhesive R&D teams, anything that provides toughness and chemical resistance sees quick adoption in the automotive or aerospace sectors. It’s the sulfur-nitrogen bond doing the heavy lifting, lending durability that carbon-only compounds can’t match.
Chemists don’t get everything for free. Thiomorpholine often comes with a noticeable odor and needs careful handling. Its synthesis sometimes generates toxic byproducts. Traditional methods often rely on haloethylamines and sodium sulfide, producing smelly, hazardous wastes. That’s an environmental headache in crowded industrial parks.
Safer, greener chemistry starts becoming more important as regulations tighten and communities demand better air quality. Some researchers have started designing alternative routes, using cheaper and less hazardous raw materials. Catalysts based on earth-abundant metals cut down on harsh conditions. Companies moving in this direction often find not just an easier regulatory path, but also less waste hauling and lower costs.
Pharma folks tend to stay quiet about their hottest new syntheses for fear of copycats, but the academic world spreads ideas faster. One approach that’s piqued interest involves microbial fermentation — getting a genetically modified bug to churn out sulfur- and nitrogen-rich rings from renewable feedstocks. A big breakthrough here could change the economics for thiomorpholine overnight.
Big things often grow from small, overlooked starting points. Thiomorpholine, with its adaptable little ring and strong elemental mix, shows how a simple compound can push big changes in medicine, industry, and materials science. If researchers and manufacturers keep finding cleaner, safer ways to make and use it, more industries will take notice. And sooner or later, everyone from patients to engineers starts reaping the benefits.
Thiomorpholine lines up with the formula C4H9NS. It comes from a six-membered ring, joining four carbon atoms, a nitrogen atom, and a sulfur atom. It might sound complicated, but in practice, this ring is actually a workhorse, quietly helping out behind the scenes in labs and chemical plants.
Digging deeper into basic chemicals like this, you figure out the building blocks behind medicines and new materials. Thiomorpholine’s ring with both nitrogen and sulfur gives it a special knack for blending in or reacting where other molecules just bounce off. That’s not a small deal in drug development labs or even when making special plastics.
This chemical is a sibling to morpholine, which swaps sulfur for an oxygen atom. That small change gives thiomorpholine its own twist, especially in how it plays with other chemicals. I remember during my own chemistry days how a slight difference in a single atom could change how a reaction went. That old classroom smell—the one you get when mixing up these sulfur-containing compounds—always stuck with me. It signaled something potent, something that could lead somewhere interesting.
Knowing C4H9NS gives a quick read on what you’re dealing with. A formula isn’t just numbers and letters—it’s a shortcut to understanding what might happen if you heat it up, mix it with acids or bases, or try to link it with other compounds. For folks working in pharmaceuticals, a sulfur-nitrogen ring signals possible links to drugs that target the brain or treat infections, since those atoms set up all sorts of nifty chemistry for attaching other functional groups—sometimes making drugs work better, sometimes helping them get to the right spot in the body.
Plenty of basic chemicals bring puzzles about waste, toxicity, and long-term effects. Thiomorpholine is no exception. While handling it, gear up—gloves, goggles, and a mask if the vapor gets too strong. Sulfur-based organics often come with that sharp, eggy smell, a warning not to drop your guard in the lab. It pays to know what you’re breathing and what might happen if a reaction starts running out of control.
Chemical waste and runoff don’t just disappear. Making sure these compounds get broken down in waste treatment, or recaptured and recycled, keeps them from seeping into water supplies. I’ve seen how even low levels of forgotten solvents can cause headaches for both facilities and neighbors down the line.
It helps to look for alternatives or ways to use less hazardous reagents. Labs now lean into using smaller amounts, recycling where possible, or swapping in greener solvents and reactants. Sometimes, researchers try to design similar molecules without the sulfur, or with different ring structures, aiming to keep the useful properties but lose some of the risk. Keeping one eye on the hazards keeps science from biting back later.
C4H9NS might not make headlines, but that basic formula opens doors—if you know how to use it wisely and carefully.
People don’t sit around their dinner tables talking about thiomorpholine. You don’t hear it in the news every day, so most of us don’t know it even exists unless chemistry plays some part in our jobs. Still, in certain manufacturing circles, thiomorpholine comes up. It gets used as a building block in pharmaceuticals, rubber chemicals, and pesticides. It pops up wherever someone wants a little sulfur and nitrogen packed into a single, ring-shaped molecule. This quiet presence makes it worth asking: does thiomorpholine carry any risk for the people who make it, use it, or might stumble across it by accident?
People working with thiomorpholine in a chemical plant might spend hours around containers, piping, or reactors that hold the stuff. If a person opens a drum or starts a line-cleaning job, there’s always a chance for spills, vapors, or splashes. I remember touring a chemical factory where a small leak in a line could leave an odor in the air that pinched the nose. Chemicals like thiomorpholine let folks know they’re there through smell and the way they make your eyes sting.
Studies report that thiomorpholine can irritate the eyes, skin, and respiratory tract. If you breathe in the vapors, your lungs may start to itch. A bit of thiomorpholine touching bare skin might turn it red or cause a rash. Manufacturers log reports of headaches, dizziness, and, with heavy exposure, nausea. Nothing here feels surprising—sulfur-containing compounds often leave people feeling rough, at least for a few hours.
No chemical stands as “harmless” when folks handle it in drums, vats, or buckets. As with painting or household bleach, the real problem shows itself where caution fades. Thiomorpholine jars memories of cheap rubber gloves melting on a factory line if left in contact too long. Most factory workers use goggles, gloves, and sometimes respirators for a reason—they know exposure to something unfamiliar means playing it safe.
There’s one thing to keep in mind: long-term health studies haven’t stretched over decades for thiomorpholine, so there could be risks lurking that we don’t understand fully. Animal tests suggest higher doses could lead to kidney or liver changes, but those studies use levels way above what a worker would see with good factory controls. Nobody recommends casual or repeated exposure—not for thiomorpholine, not for anything in its family.
The lesson feels simple after watching any real chemical workplace in action. Companies keeping their workers safe usually win in the long run—fewer lost-man-hours, smaller insurance claims, better morale. It matters that places using thiomorpholine follow basic industrial hygiene: keep levels low through ventilation, pipe leaks away, enforce glove and goggle rules, and train people so they know what they’re up against. Clean hands before lunch. Showers at the end of a shift. Small steps go a long way.
Whenever I hear stories about chemical accidents—rushed jobs, spilled solvent, fumes in a closed room—it drives home the importance of planning and experience. No one wants surprises, least of all those whose job is to stand next to barrels and lines all day.
If you work with thiomorpholine, or live near a plant that uses it, let yourself ask questions. Easy access to safety sheets, real training, and an open door to report leaks or headaches helps everyone in the long run. Regulators can help by pushing for honest risk disclosure and encouraging companies to look for safer substitutes where possible.
The world will always use chemicals to make medicine or manufacture rubber, but people deserve both progress and safety. A little caution, shared information, and respect for what we don’t fully know yet keeps both health and business on solid ground.
Anyone working around chemicals for a stretch learns to respect bottles that look ordinary on the outside but pack volatile surprises. Thiomorpholine doesn't shout its hazards from the rooftops, yet it holds enough dangers if stashed carelessly. I’ve watched labs skip the manual and pay for it with ruined supplies, ruined equipment, or worse. Storing thiomorpholine calls for some basic discipline, less about fancy systems and more about routine and respect.
Thiomorpholine shows up as a colorless liquid, with a sharp smell that reminds some of sulfur or ammonia. Its odor can stick in your mask during a long day. The stuff flares up with the wrong spark and eats away at common plastics. Over time I’ve noticed folks get complacent, tossing it onto a general chemical shelf, unsealed. That’s trouble waiting to happen.
The facts are simple: thiomorpholine can catch fire, boils at 135°C, and gives off toxic fumes during decomposition. Inhaled vapors hurt your lungs, and some tests suggest the liquid causes skin corrosion. No one wants to deal with chemical burns or a visit from the hazmat crew over a shortcut.
Keeping thiomorpholine safe calls for a cool, tightly sealed bottle, stashed in a spot with sturdy ventilation. In my experience, placing it eye-level on a shelf just for flammable organics, well away from acids and oxidizers, means spills won’t set off a chain reaction. Storing it in glass or compatible plastic, not a reused soda bottle someone grabbed in a pinch, keeps the contents stable over weeks or months.
Temperature plays a quiet but deadly role. Too much heat and thiomorpholine can build up pressure, pop a cap, or spill. Temperatures around 15–25°C keep things steady; freezer isn’t better, because condensation can creep in during thawing and mess with the chemical’s integrity. I keep an inexpensive thermometer taped beside the cabinet as a reminder.
Shortcuts don’t only come from laziness, sometimes it’s confusion over what something can really do. Training newcomers on proper segregation and showing scars, both on hands and benchtops, goes a long way. Clear labeling, separate secondary containment (like a polyethylene tub), and up-to-date chemical logs all help prevent accidents. One time we nearly lost a whole season’s research when a leak corroded shelving; after that, we double-checked every seal and kept spills kits handy.
Labeling “store in a cool, dry place” on the bottle means nothing without follow-through. It helps to mark each storage location with clear, bold tags. Simple routines—checking lids, inspecting visual clarity of the liquid, tracking quantity—add another layer of safety. Regular audits by a second set of eyes spot mistakes early.
If your workplace doesn’t invest in ventilated chemical storage cabinets, advocate for it. Tackling these basics doesn’t just keep inspectors happy; it means everyone goes home whole. Respect for thiomorpholine, or any chemical, comes from these lived lessons and practical guards more than textbook warnings. Standard care saves supplies and keeps labs moving, but more importantly, it keeps people safe.
Thiomorpholine isn’t a household name, but it plays a bigger role than most people notice, especially in pharmaceuticals. Some cough medicine ingredients get their backbone from this sulfur-containing compound. Making it means wrangling with both chemistry and some old-school laboratory craft, and it’s a process with a story worth telling.
In the lab, the most direct route starts by reacting a halogenated ethylamine (like 2-chloroethylamine) with a sulfur source, usually sodium sulfide. Think of it as bringing together bricks (the ethylamine) and mortar (the sulfur) to build a ring. With a bit of heat and care, the reaction ties the four carbons, one nitrogen, and one sulfur into a six-membered ring. Honestly, it sounds straightforward on paper but can get hairy. Both reagents bring their own hazards—chloroethylamine is pretty toxic and sodium sulfide isn’t much kinder to the skin. Chemistry rarely rewards those who forget their gloves.
A more modern touch swaps out the above ingredients for diethanolamine and a thionating agent, most often phosphorus pentasulfide or Lawesson's reagent. Here, the diethanolamine serves as a flexible scaffold, and the phosphorus reagent gently replaces oxygen atoms with sulfur, nudging the molecule to curl into that signature ring shape. This method caught on because diethanolamine is less nasty to handle and easier to source than its halogenated cousin. Still, phosphorus pentasulfide comes with its own quirks: it stinks, it smokes, and it demands respect in a chemical hood. More than one chemist has learned those lessons the hard way.
I’ve seen enough labs where attention to solvent choices decides who leaves on time and who stays late cleaning up an avoidable mess. Both methods usually run in polar solvents—water, ethanol, or DMF. No surprise, these solvents make the reactants dissolve and swirl around just right, speeding up the ring-forming action. The catch: what you choose ends up shaping both your yield and how much cleanup the process throws at you later.
Making thiomorpholine might not demand full-scale factory-size equipment, but it does take respect for safety. In small companies, keeping chemical waste low often trumps prestige or tradition in choosing a synthesis route. Managers who listen to the chemists about why phosphorus pentasulfide stinks less than a sodium sulfide spill usually spend less on complaints and repairs.
I’ve watched chemists run small tests with microwave heating and water-based solvents, partly to chop down heating times and snip out extra toxic byproducts. Sometimes these approaches help, sometimes they just introduce fresh headaches. Yet, pressure keeps mounting to move away from harsh reagents and heavy metal waste. Think about the cost: scrubbing out sulfur or phosphorus byproducts racks up real dollars, not to mention environmental headaches later.
So, more and more labs tinker with biocatalysts or recyclable sulfur sources. Some R&D teams dream of shifting everything to flow chemistry: reactants trickle through tiny reactors, heat stays local, and spills shrink to nearly zero. Hardware investments sting, but for process chemists and environmental teams, these setups start looking like long-term bargains.
With global demand climbing, cleaner routes for thiomorpholine aren’t just some green wish—they’re practical goals for both safety and budgets. Pushing for fewer stinky reagents, milder conditions, and softer handling practices shapes how the next generation of chemists learns this business. Safer, simpler syntheses win over everyone who has spent late evenings in the lab fixing sloppy messes.
| Names | |
| Preferred IUPAC name | Thiomorpholine |
| Other names |
4-Thiahexahydro-pyridine 1-Thiomorpholine Thiomorpholine Tetrahydro-1,4-thiazine |
| Pronunciation | /θaɪ.oʊˈmɔːrfəˌliːn/ |
| Identifiers | |
| CAS Number | 123-90-0 |
| Beilstein Reference | 2811223 |
| ChEBI | CHEBI:36983 |
| ChEMBL | CHEMBL156356 |
| ChemSpider | 21526 |
| DrugBank | DB02709 |
| ECHA InfoCard | 100.018.884 |
| EC Number | 212-995-2 |
| Gmelin Reference | 82259 |
| KEGG | C19111 |
| MeSH | D013868 |
| PubChem CID | 12021 |
| RTECS number | WS5075000 |
| UNII | Y6M8J4PVK2 |
| UN number | 2810 |
| CompTox Dashboard (EPA) | DTXSID3020704 |
| Properties | |
| Chemical formula | C4H9NS |
| Molar mass | 119.19 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Amine-like |
| Density | 1.253 g/mL at 25 °C |
| Solubility in water | Miscible |
| log P | 0.02 |
| Vapor pressure | 0.4 mmHg (20°C) |
| Acidity (pKa) | 8.57 |
| Basicity (pKb) | 4.83 |
| Magnetic susceptibility (χ) | -49.7·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.535 |
| Viscosity | 0.96 cP (20°C) |
| Dipole moment | 2.35 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 282.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -77.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4849.6 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS05 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P280, P305+P351+P338, P304+P340, P337+P313 |
| NFPA 704 (fire diamond) | 2-3-0 |
| Flash point | 62°C |
| Autoignition temperature | 215 °C |
| Explosive limits | Explosive limits: 7–15% |
| Lethal dose or concentration | LD50 oral rat 1400 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Thiomorpholine: **2150 mg/kg (oral, rat)** |
| NIOSH | WN8400000 |
| PEL (Permissible) | PEL for Thiomorpholine: Not established |
| REL (Recommended) | 60-100 mg/m³ |
| IDLH (Immediate danger) | 100 ppm |
| Related compounds | |
| Related compounds |
Morpholine Piperazine Thiirane Tetrahydrothiopyran |