Long before laboratories became polished palaces of innovation, chemists hovered over odd-smelling mixtures in cramped rooms. Thiazole-4-carboxylic acid didn’t leap into chemistry overnight. Its backstory ties to the curious 19th-century pursuit of heterocyclic compounds. Early researchers, often stumbling through trial and error, pushed toward understanding sulfur and nitrogen blending within aromatic rings. The thiazole framework itself, born from cross-disciplinary curiosity, eventually locked a place in the history books when folks figured out the significance of sulfa drugs in medicine. For years, thiazole-4-carboxylic acid emerged as an overlooked cousin, shadowed by its more famous relatives. Progress often shows up this way: relentless, piecemeal, unspectacular until someone draws connections that matter. I see this story woven through every old chemistry journal, every weathered lab notebook dusted off after decades. Scientific developments rely on such patience—incremental steps built on scraps of previous work.
Not many folks outside of chemistry circles encounter thiazole-4-carboxylic acid’s name on a daily basis, but it shows up in more places than you’d think. The compound presents itself as a fine, usually off-white to yellowish crystalline powder, largely odorless and sometimes a little bitter. If you peer beyond the jar, you’ll find it on order sheets for specialty chemical suppliers, packed away with countless documentation and labeling stickers that reassure buyers about its identity and origin. Manufacturers often tout high purity ratings, with numbers typically falling at or above 98%. What catches the eye is not how it looks, but how quietly it supports industries needing small but meaningful molecular changes—be it in pharmaceuticals, dyes, or agrochemicals.
You can pick out thiazole-4-carboxylic acid from the rest of the thiazole crowd with a bit of practice. Its melting point usually lands somewhere around 176-178°C, which keeps it stable for most applications. The compound dissolves reasonably well in polar solvents like dimethyl sulfoxide and methanol but doesn’t budge much in water. This limited water solubility sometimes complicates process steps, especially when a formulation’s success hangs on getting every ingredient to dissolve. Chemically, the acid group on the fourth carbon draws attention for folks aiming to tweak molecules; it forms neat linkages and offers a solid handle for modifications. The molecule’s hybrid sulfur and nitrogen content plays into its slightly acidic character, with a typical pKa in the upper 3s to low 4s. These features often matter more to those mixing it into reactions than to those typing up a spec sheet.
Conversations with anyone handling specialty chemicals always circle back to specs and paperwork. Suppliers list batch numbers, assay percentages, IR or NMR spectra for confirming the compound’s identity, and pack everything with detailed safety sheets. Labs demand more than just purity—they want to know about residue on ignition, loss on drying, and heavy metal content. These technical details might sound dry to most, but they play a crucial role in reducing the chances of ruining an experiment downstream. Someone dropping a bottle of thiazole-4-carboxylic acid into a project wants to spend time solving actual problems, not tracing contaminants. Clear labeling coupled with precise documentation keeps everyone honest, including customs officials, researchers, and regulatory reviewers.
Digging into synthesis methods shows how much persistence chemistry asks from its practitioners. For thiazole-4-carboxylic acid, early routes often relied on cyclization reactions—pairing α-bromopyruvic acids with thioamides, for instance. Yields started out unimpressive until process tweaks and alternative starting materials improved matters. The choice of solvents, reaction temperature, and even stirring speeds changed outcomes. Over the years, teams have adopted greener routes where possible, trading dangerous reagents for milder alternatives. Microwave-assisted syntheses, flow chemistry, and enzymatic modifications started showing up at conferences. Each breakthrough aimed to shave hours off reaction times and push purity higher, making scale-up not just possible but cost-effective. Even now, chemists keep their ears open for a faster, gentler way to build these structures—always hunting for the edge.
The fourth carbon on the thiazole ring gives chemists a playground for attaching other functional groups. Carboxylic acids in organic chemistry make great scaffolding—activate it as an acid chloride, tether it to amines via amide bonds, switch it out for esters, or use it as a springboard for constructing even more elaborate molecules. In medicinal chemistry labs, this acid often finds itself morphed into prodrugs or linked to active pharmaceutical ingredients through clever conjugation. On the industrial side, thiazole-4-carboxylic acid helps build crop protection agents and specialty dyes. Its unique electronic nature tweaks properties of whatever it attaches to, shifting solubility or biological activity in directions plain thiazole can’t offer. This adaptability makes it valuable for researchers chasing improved performance across wildly different domains.
Step into a supplier catalog, and you’ll bump into a handful of names describing the same thing: Thiazole-4-carboxylic acid, 4-thiazolecarboxylic acid, or even 4-carboxythiazole. These labels sometimes vary based on regional naming conventions or registration requirements. Chemical Abstracts Service (CAS) tacks on a specific registry number—3973-08-8, for those checking. This isn’t just trivial bureaucracy; a researcher needs to make sure what’s on the bottle matches what goes into the flask. Mixing up similar-sounding ingredients could spoil months of planning. Knowing these synonyms, and relying on catalog identifiers and safety data sheets, keeps confusion at bay, especially as supply chains stretch across continents.
Nobody wants to risk injury or contamination in their pursuit of discovery, so safety protocols around thiazole-4-carboxylic acid deserve attention. The compound isn’t especially notorious compared to stronger acids or toxic solvents, but common sense prevails—lab coats, gloves, safety goggles on at all times, and working within a well-ventilated environment. Material safety data sheets flag the irritant potential of the compound; skin, eyes, and mucous membranes need to stay protected. Spills call for swift cleanup with absorbent material, and disposal follows local chemical waste guidelines. Mechanical ventilation and clear labeling stop small errors from becoming emergencies. Training, hazard communication, and regular drills keep operations safe for everyone in the room.
Few chemicals stay relevant without finding meaningful jobs. Thiazole-4-carboxylic acid gets work in several busy sectors. In drug discovery, it helps as a building block for synthesizing antibiotics, anti-inflammatory agents, and metabolic modulators. Some of its derivatives push into agrochemicals, where they help safeguard crops against fungal or bacterial threats. The dye industry taps it for molecules that produce unique shades or respond well to certain textile treatments. Analytical labs sometimes use it in reagent kits, especially when specific reactivity offers an experimental advantage. Each field leans hard on the robustness of the core thiazole ring, while the carboxylic acid group steers reactivity and compatibility. Companies planning product launches or process improvements look for such versatile starting points, as these can streamline production and cut costs.
Focusing on thiazole-4-carboxylic acid in R&D spaces makes sense for groups searching for unique biological activity or reliable synthetic routes. Academics examine its interactions with enzymes, its behavior as a modulator or scaffold in medicinal chemistry, and its ecological impact as breakdown products enter water systems. I’ve noticed a steady parade of journal articles each year that detail new derivatives or improved methods for connecting this ring to anything from peptides to polymers. It’s not unusual to see startups tinkering with the scaffold, trying to build patent portfolios or pitch next-generation therapies. Research funding tends to gravitate toward these multipurpose intermediates, especially as customization drives the pharmaceutical and materials industries forward.
Toxicologists devote much effort to predicting and confirming how thiazole-4-carboxylic acid and its derivatives interact with living systems. So far, acute toxicity hasn’t drawn major alarm, but animal studies look at longer-term effects, organ distribution, and metabolic products. Water solubility, though limited, drives interest in how waste products from its synthesis or use drift into groundwater and the food chain. Regulatory bodies demand rigorous testing, sometimes holding up commercial use for years. Having clear data on toxicity and metabolites helps public confidence and supports downstream development. Anyone trying to push a new product through approval knows this part can bottleneck progress, but skipping it courts disaster.
Looking ahead, demand for customizable building blocks like thiazole-4-carboxylic acid keeps rising. Drug discovery, crop science, and specialty materials show no signs of losing interest in flexible, functional molecules with a solid track record. I expect to see more innovation in sustainable methods for large-scale synthesis, less hazardous reagents, and greener solvents. Additive manufacturing and automated synthesis could bring costs down, making this and related compounds more available to a broader market. Researchers and industrial chemists will keep picking it up, stretching the boundaries of what this humble ring can unlock. The quest remains the same: harness molecular structure for real-world impact, with thiazole-4-carboxylic acid at the core of countless new projects across pharmaceutical and chemical manufacturing landscapes.
Every now and then, a chemical with a name straight out of a spelling bee pops up and turns out to play a bigger role in everyday life than most folks expect. Thiazole-4-carboxylic acid, or T4CA for short, is one of those behind-the-scenes ingredients in science labs and pharma plants that's tougher to spot in your medicine cabinet but has quiet influence over a range of products.
Thiazole-4-carboxylic acid often shows up as a building block for drug development. Scientists prize it because the thiazole ring—a five-membered structure with both sulfur and nitrogen—brings a lot of versatility when stitched together with other parts of a molecule. Chemists can tinker with attached groups, opening the door for all sorts of new compounds. Drug researchers love using these rings when crafting antibiotics, cancer treatments, and even medicines for diabetes.
Anyone who’s spent time in a college research lab has likely seen these powders getting weighed and mixed, sometimes with little more fanfare than a spoonful of sugar in a mug of coffee. There’s a quiet thrill in watching something plain—just a pale yellow solid—turn into a crucial ingredient for a medicine that ends up helping folks fight an infection or tamp down inflammation. Real progress often comes from piecing together simple building blocks like this.
Beyond medicine, T4CA also gets invited to the party in agrochemical synthesis. Crop scientists tap it when developing new pesticides or herbicides. By slipping this molecule into the recipe, they’re able to fine-tune the effectiveness or safety of a new chemical. This matters for anyone who likes tomatoes on their sandwich or wheat in their bread. Strong crops and healthy fields owe quite a bit to unsung chemical ingredients like this, even if no one ever sees their names on the final bag of fertilizer.
Finding a steady supply of reliable thiazole-4-carboxylic acid presents its own challenges. Purity makes a big difference—a batch with too many impurities can send an entire research project off track. I’ve watched lab budgets balloon when delays or low-quality stock forced us to order rush shipments. Fake or inferior chemicals drain time and money that could’ve gone toward breakthroughs.
Plenty of folks assume modern labs run like well-oiled machines, but the reality is often closer to a diner kitchen. Order up a key ingredient and hope it arrives in time—and pray it doesn’t spoil the recipe. The fight for better sourcing means some manufacturers have doubled down on their quality controls and invested in tighter oversight. Tracking down a reputable supplier takes effort but pays off when the new crop protection formula or latest medical compound actually works as advertised.
Improved transparency along the supply chain can help researchers and companies move faster. Lab managers should have direct, plain-English conversations with suppliers about origin and documentation. Third-party testing offers another layer of trust, especially for small operations without fancy equipment to check every shipment.
There’s also potential to develop greener and more cost-effective ways to produce thiazole-4-carboxylic acid. Traditional methods use a lot of solvents and energy, which piles up waste. Shifting toward cleaner chemistry could cut costs for everyone, shrink the paperwork mountain, and make more of these valuable building blocks available for research that matters both in the fields and on pharmacy shelves.
At the end of the day, thiazole-4-carboxylic acid isn’t just another mouthful of a name in the chemical catalog. It’s a reminder that progress often starts with the basics—simple tools, good communication, and constant efforts to do the job a little better every year.
Plenty of folks enter the world of chemistry thinking it’s about mixing colored liquids and hoping for fireworks. In reality, it often comes down to the cold, hard numbers—like the molecular weight of a compound. Take thiazole-4-carboxylic acid. Its molecular weight stands at 129.12 g/mol. Kind of an unsung, everyday figure in the chemistry lab, but definitely one that packs a punch in what it unlocks.
I’ve spent my fair share of time weighing out powders in the lab. Making mistakes on molecular weights turns success into wasted hours and blown budgets. 129.12 isn’t just trivia. It’s what lets you scale up a reaction and actually know what you're working with—not just guessing, but running experiments that save time and money.
Now, thiazole-4-carboxylic acid, with its five-membered ring and sulfur and nitrogen combo, plays its role in building more complex molecules, whether that's for potential medicine or clever new materials. The molecular weight is your ticket to figuring out the right proportions, the solvent volumes, the reduction yields, and even the leftover waste to dispose of responsibly.
Let’s break it down once and for all without the chemistry jargon overload. Thiazole-4-carboxylic acid is made up of five carbons, three hydrogens, one nitrogen, one sulfur, and two oxygens. Each of those atoms has its own tiny weight. Add them up and boom—129.12 grams per mole. Every batch ordered, every compound weighed, this number keeps you honest.
It still baffles me that this basic figure keeps popping up in forums and group chats. Maybe online databases seem intimidating. Maybe it’s the way some textbooks hide these numbers between long-winded explanations. Or maybe folks want to double check their numbers, so small mistakes don’t turn into big problems.
In my early days, I once calculated a whole synthesis based off a guessed weight. The whole project fizzled, and I learned quickly that these numbers can’t get glossed over. Chemists building new medicines or researchers doing routine tests all need the same thing: accurate molecular weights, so results make sense and can actually be trusted.
Some labs use expensive machines to verify compound identity. Most just check and re-check their calculations. Nobody in the lab wants to be that person who wasted precious reagents because they got the molecular weight wrong. It’s not about pride—it’s about real results and not wasting resources.
One way to make sure fewer mistakes happen? More straightforward, accessible resources. There’s a real need not just for open access to data, but for practical guides that don’t require a chemistry degree to make sense of. Also, chemistry teachers can do plenty by weaving routine calculations into daily lessons and guiding students through hands-on examples instead of piling on theory.
With open discussion and more knowledgeable sharing, these numbers turn from background noise into the backbone of smoother, more reliable scientific work. From my time in science classrooms to industrial settings, I’ve seen confidence grow when everyone has the right figures handy.
Most folks staring at a jar labeled “Thiazole-4-Carboxylic Acid” don’t get a warm, fuzzy feeling. This powder packs more rules than you’d think. Sometime back, I walked into a shared lab fridge and found chemical jars jammed behind someone’s lunch. Bad idea all around. These compounds often come with storage quirks that folks skip out of haste or laziness.
Heat, light, and moisture can wreck Thiazole-4-Carboxylic Acid faster than you can read the label. I’ve seen whole batches clump up or break down, all from a missed seal or busted box. The stuff holds up better at cool room temperatures — not the hot, stuffy cupboard near pipework. Best bet? Move them somewhere dry and cool, away from wild swings in temperature.
Sunlight has this sneaky way of transforming useful substances into useless junk. Instead of leaving jars out, grab an amber bottle or slide them into a container that blocks light. I once left a similar compound on a sunlit shelf for just a few days and opened it to find a suspiciously off-color powder — lesson learned.
Regular containers let humidity creep in. This acid in particular loves picking up water from the air. If humidity rises, so does your risk of nasty surprises: ruined samples, lost results, safety woes. Always screw on those lids tight. A desiccator seems old-school until you realize one open jar could spoil weeks of effort.
Reaching for a jar and realizing you can’t read the label is a shortcut to mistakes. Permanent markers wear off in the cold, so slap on a durable label with the chemical name and date received. I watched a colleague misplace a compound once, only to unearth it later during a shelf clean-out. Bad tracking can trip up even the most careful team.
Reusing food jars or random plastic bottles doesn’t cut it. Only use tight-seal glass or HDPE containers free from cracks. Some plastics leach or react over time. Labs that skimp here end up with tainted samples or, worse, safety risks. Don’t forget to store it away from strong acids or bases. Unexpected reactions start quietly, often from a stray drop or forgotten spill.
The temptation to cut corners on storage comes from time pressure and clutter. Every time I think of hurried storage, I remember the day a fume hood filled with acrid stench — caused by someone skipping simple steps. Consistent routines make the difference between stable, usable powder and problems. Make a storage checklist or quick reminder cards if you must.
Proper storage isn’t about bureaucracy or needless hassle. It keeps Thiazole-4-Carboxylic Acid reliable, workers safe, and budgets intact. Talk with your team, review those labels, check for tight lids, and ditch anything with mystery smudges. One small effort in the lab can save everyone a world of headache — and keep that acid where it belongs: quiet, stable, and ready for the right moment.
Whether you’re working in a university lab or a pharmaceutical plant, the chemicals poured into a process directly shape the results. Purity stands at the center of chemical work. Thiazole-4-carboxylic acid finds a spot in drug synthesis, research, and dye formulation, among other applications. The right batch can decide if your experiment succeeds or your product meets regulatory checks.
It doesn’t take long in a laboratory to recognize how even slight impurities mess with outcomes. If you’ve ever had a reaction fail for reasons unclear, contaminated reagents sit high on the list of suspects. With compounds like thiazole-4-carboxylic acid, aiming for a high-purity grade becomes more than just industry tradition—it turns into an everyday safeguard.
Different projects call for different levels of purity. In my own experience, chemical suppliers often provide thiazole-4-carboxylic acid in several grades. You can easily spot listings for “technical,” “laboratory,” and “analytical” purity. These terms may look similar but reflect very different starting points. Technical grade works fine for early-stage synthesis where cost control matters more than trace contaminants. If you need something reproducible and clean for more crucial steps, laboratory grade offers more reliability. Analysts looking for trace-level detection or high-sensitivity work prefer analytical grades, often above 98% purity.
Even within a single grade, small fluctuations can become a headache. I’ve handled products labeled 97% pure only to see actual reports come in at 95.9% or a bit higher, depending on the batch. Regulatory filings or scale-up processes push for documentation with every shipment. Purity isn’t just about what’s present—what’s absent matters too, as regulatory challenges often arise from specific impurities like heavy metals or solvents rather than the parent compound.
More purified chemicals cost more. You pay extra for every step the compound goes through—from crystallization to re-crystallization, from advanced filtration to handling in spotless facilities. Budgets do battle with project goals every day. I’ve seen projects delayed not by the science, but by wrangling over which grade is strictly necessary versus which is “nice to have.” A tiny percent jump in purity can double the price, yet the payoff returns as savings downstream thanks to cleaner data or product batches that pass inspection.
Sourcing also plays a role. Not every vendor stocks every grade at all times. If rare or ultrahigh-purity thiazole-4-carboxylic acid runs out, timelines stretch and costs jump further. Latin American or Asia-based labs sometimes lean on local suppliers, while US or European buyers take to bigger chemical distributors with ISO certifications and batch-level documentation. Each source may use slightly different testing methods, making cross-comparisons tricky. A little digging around safety datasheets and certificates of analysis becomes routine before placing any order.
Every lab should keep a record not just of the chemicals it buys but the specific grades in use. Comparing project outcomes over time tells you if the penny-pinching on purity paid off or if switching to a higher grade would have solved recurring problems. Building a relationship with reputable suppliers also helps, as they’re often open to sharing batch histories, third-party testing, and details down to solvents or synthesis side-products.
Sometimes the solution doesn’t even involve switching grades. Fresh handling protocols, better storage, and clear labeling can cut cross-contamination and keep impurity risks low. Thiazole-4-carboxylic acid’s purity may look like just a technical detail in a catalog, but those percentages show the difference between spent resources and reliable output. Anyone running reactions or scaling up products knows how these chemical choices shape results long after the invoice is paid.
Thiazole-4-carboxylic acid catches the eye of chemists and drug designers. The question about its chemical formula is simple, but the story behind it matters just as much for people who deal with synthesis, testing, or just academic interest in heterocyclic compounds. Thiazole rings show up in many places—therapeutics, bioactive molecules, and even dyes. For plenty of researchers, the structure isn't just a drawing on a paper, but the backbone of inventions in medicine and industry.
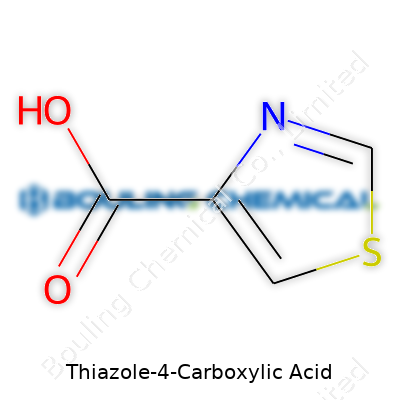
The chemical formula for thiazole-4-carboxylic acid: C4H3NO2S. It seems simple—just five elements, lined up in a straightforward way. To someone who dabbles in organic chemistry, those numbers reveal how the molecule weaves together. Four carbons and one nitrogen make the aromatic thiazole ring, and sulfur jumps right into the five-membered structure. At the fourth position, a carboxylic acid group—COOH, familiar from school chemistry—hangs off the ring.
A molecular formula isn’t just trivia. It tells you exactly what’s possible with a compound. Drug developers care because small changes in formula or placement can change a molecule from a life-saving medicine to something completely inactive. In the real world, misreading a formula can send weeks of lab work down the drain. I remember working on heterocyclic scaffolds in a research stint; a single placement error delayed our synthesis by a month. Thiazole-4-carboxylic acid sits as a prime candidate for building blocks because its formula spells out multiple functional groups and aromatic behavior, and both come in handy for pushing reactions in the right direction.
Working with thiazoles, the first thing to check is solubility. The formula covers a polar group (the carboxyl) and the less polar ring (the thiazole). Chemists see these clues and pick their solvents. Water won’t do everything. Mix it with something like DMSO or ethanol, and the compound starts behaving. Those who run reactions or plan crystallizations know the role each element plays in sticking crystals or separating layers in a funnel.
Challenges crop up anytime you have sulfur and nitrogen together in a ring. They like to react with odd things in the air or the bottle, sometimes changing over time. It isn’t just theory—I’ve tossed out poorly sealed samples after oxidation turned them yellow. The formula also guides chemists in picking purification techniques. With an extra oxygen in the carboxylic acid, acidity can become both a benefit and a pain, depending on the desired result.
Industrial labs and startups alike keep searching for better ways to turn these molecules into medicines, sensors, or agricultural chemicals. Focusing on the formula, teams can tweak parts of the molecule—maybe swap in a fluorine or extend the chain—and each atom matters. Patents often hinge on small changes that start from this base formula. Instead of generic modifications, targeted substitutions offer a path toward new bioactive compounds that sidestep resistance, treat infections, or light up in biosensors.
The formula, C4H3NO2S, remains the foundation. Young chemists ask for this detail on practicals; seasoned researchers use it to predict spectra, solubility, and function. Whether you’re dealing with the theory or the messy business of separating a new compound from byproducts, the simple formula points the way—one small, sulfur-rich ring at a time.
| Names | |
| Preferred IUPAC name | 4-thiazolecarboxylic acid |
| Other names |
4-Thiazolecarboxylic acid Thiazol-4-carboxylic acid 4-Thiazolylcarboxylic acid Thiazole-4-carboxylate |
| Pronunciation | /θaɪˈæzoʊl fɔːr kɑːrˈbɒksɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 3973-08-8 |
| Beilstein Reference | 110937 |
| ChEBI | CHEBI:36835 |
| ChEMBL | CHEMBL285002 |
| ChemSpider | 20722679 |
| DrugBank | DB08722 |
| ECHA InfoCard | echa.europa.eu/substance-information/-/substanceinfo/100.021.226 |
| Gmelin Reference | 64894 |
| KEGG | C06397 |
| MeSH | D013857 |
| PubChem CID | 107061 |
| RTECS number | XN8225000 |
| UNII | SWG9J6703F |
| UN number | UN3335 |
| CompTox Dashboard (EPA) | DTXSID4077608 |
| Properties | |
| Chemical formula | C4H3NO2S |
| Molar mass | 127.12 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.43 g/cm³ |
| Solubility in water | Slightly soluble in water |
| log P | 0.12 |
| Vapor pressure | 0.0000145 mmHg at 25°C |
| Acidity (pKa) | 2.3 |
| Basicity (pKb) | 5.10 |
| Magnetic susceptibility (χ) | -48.0 × 10^-6 cm³/mol |
| Refractive index (nD) | 1.640 |
| Dipole moment | 3.77 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 167.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4557 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS05 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P273, P280, P302+P352, P305+P351+P338, P312, P332+P313, P337+P313, P362+P364 |
| Flash point | 173.9 °C |
| Autoignition temperature | Autoignition temperature: 410 °C |
| Lethal dose or concentration | LD50 Rat oral 1,200 mg/kg |
| LD50 (median dose) | LD50 (median dose): >5000 mg/kg (oral, rat) |
| NIOSH | SN8750000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg |
| Related compounds | |
| Related compounds |
Thiazole Thiazole-2-carboxylic acid 2-Aminothiazole Thiazole-4-carboxamide 4-Methylthiazole Thiazole-5-carboxylic acid 2-Bromothiazole Benzo[d]thiazole-2-carboxylic acid |