People have been tinkering with thiamorpholine in labs since the late 20th century, always hunting for new ways to bring heterocyclic chemistry into practical life. The earliest reports show chemists in Europe experimenting with sulfur-containing piperidine analogs, looking for changes that sulfur atoms might bring to basic molecular frameworks. As researchers tried to stretch the boundaries of medicinal chemistry and specialty polymers, thiamorpholine entered synthesis routines, earning its place beside more familiar heterocycles like morpholine and piperidine. Experience tells us that as soon as a molecule crops up in one field—synthetic intermediates for agrochemicals, pharmaceuticals, or even as a ligand in catalysis—curiosity spreads and real progress starts to happen. Throughout the last few decades, patent filings and journal articles slowly stacked up, reflecting a steady march from novelty to genuine utility.
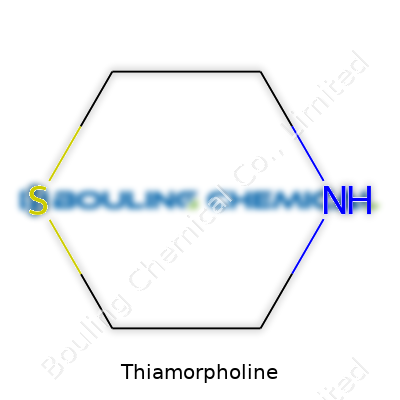
Thiamorpholine is a saturated, six-membered ring with a sulfur atom and a nitrogen atom sitting across from each other. That S-N pairing distinguishes it from regular morpholine, lending the ring a certain versatility in chemical behavior. Most people first meet thiamorpholine in a bottle labeled for research use, loaded with a thick, less-than-pleasant smell. Whether as a colorless to pale yellow oily liquid, or as a stabilized salt when folks want a bit more handling comfort, the stuff introduces itself as a specialty chemical with big ambitions in synthesis and specialty formulations. Major suppliers usually provide it in high purity, clearly targeting advanced chemical research and pilot-scale ventures.
Thiamorpholine usually flows as an oily, colorless to yellowish liquid under normal temperatures. It boils above 200°C, which puts it far from the volatility of low molecular weight amines, and the molecule dissolves nicely in water and polar organic solvents. With a molecular weight hovering near 119 g/mol, thiamorpholine’s density and viscosity make it somewhat tricky to handle in cold labs or open benches. The sulfur atom in its ring brings extra polarizability and a tendency for the whole molecule to participate in both hydrogen bonding and pi interactions, steering the chemistry in ways you don’t get from plain morpholine or piperidine. This physical character—dense, tenacious, slightly odorous—reminds me of so many thiols and thioethers found lurking in chemical storerooms.
Walk into a stockroom and you’ll see thiamorpholine labeled by its CAS number (123-92-2), purity—usually above 98%—and lot number for traceability. Most bottles also include flash point (usually around 95°C), boiling and melting points, and storage advice: keep sealed, out of sunlight, away from oxidizers. You’ll likely spot warnings about the smell, potential toxicity, and recommended PPE—belt, suspenders, and gloves. Documentation from suppliers offers an assay by GC, calculated water content, and details on storage stability. Plenty of long-time chemists, including myself, appreciate a clear specification sheet; it sets expectations and reduces surprises in the lab or pilot plant.
Labs typically synthesize thiamorpholine by reacting 1,2-dichloroethane with thiourea to form a thiouronium salt, then cyclizing that intermediate with a base to close the ring. Some routes start with ethylene oxide and hydrogen sulfide followed by amination, but the dichloroethane method stands out for simplicity and scalable yields. Purification usually happens by vacuum distillation, stripping out low-boilers and heavier side products, until the desired purity sets thiamorpholine up for subsequent reactions. I’ve run a few of these preps myself; though the process often involves pungent intermediates, careful distillation tames even the roughest concoctions, and the learning curve flattens once you get the hang of sulfur chemistry’s quirks.
Thiamorpholine takes the spotlight when people need a building block for further functionalization. The nitrogen and sulfur give access to a world of modifications: alkylation, acylation, oxidation, and ring opening. For instance, N-alkyl thiamorpholines spring up easily by treating the ring with alkyl halides—giving a path to more lipophilic or tailored ligands. The sulfur atom, sitting there in the ring, opens the door to oxidation to sulfoxide or sulfone derivatives, each offering different electron distributions and physical properties. One of my favorite tricks uses thiamorpholine as a masked thiol source in fragment-based drug design. In either case, this chemistry broadens the playground for process chemists and molecular designers alike.
In the wild world of chemical nomenclature, folks might call this compound Tetrahydro-1,4-thiazine, 1,4-Thiazane, or even trimethylene sulfamide. Some catalogs just lump it under “Thiamorpholine,” but researchers need to keep an eye out for all these names when digging through literature or regulatory filings. I’ve seen generics and custom suppliers market it with obscure trade names too, particularly for formulations in specialized organic synthesis kits.
Safety officers who know their business never let thiamorpholine slip past without respect. Though not a heavy-duty poison like some organosulfur compounds, thiamorpholine can sting: inhaling vapors, swallowing the liquid, or splashing it in eyes spells trouble from irritation to toxicity. Standard operating procedures call for fume hoods, nitrile gloves, and goggles; I’ve seen what happens when someone ignores a whiff—persistent headaches and burning throats aren’t a pleasant memory. People store it in tightly sealed bottles, away from acids and oxidizing agents, since sulfur can occasionally serve up bad surprises by forming noxious gases or exothermic mixtures. Spills get scooped up with absorbent materials for hazardous waste disposal, never down the drain.
As a synthetic intermediate, thiamorpholine punches above its weight in medicinal chemistry, agrochemical lead discovery, and polymer science. Medicinal chemists eye its unique ring system for metabolic stability, with the sulfur atom tweaking bioavailability and transport. Crop protection researchers turn to it for modifying active agents, aiming for improved field persistence. In the world of specialty polymers, you sometimes spot derivatives woven into backbone chains, drawing on the sulfur’s potential for flexibility or crosslinking. It’s even popped up in analytical chemistry as a ligand in certain metal-catalyzed processes. Based on conversations and journals I’ve scoured, the true potential still sits untapped, mostly because thiamorpholine knowledge hasn’t trickled downstream from research benches to full industrial scale.
The R&D crowd doesn’t shy away from exploring thiamorpholine’s crossroads of reactivity. Dozens of groups experiment with its modification, looking for new antibiotics, fungicides, or probes. Some have explored coupling reactions to bolt on aromatic groups, others dangle pendant chains off the ring nitrogen or sulfur. Its ring rigidity and electron-rich character prompt teams to try it in developing new organocatalysts. While the main bulk of research hides in subscription-only journals and proprietary company files, conference posters often highlight fresh directions: analogs with increased selectivity, backbone modifications for hybrid drugs, or use as molecular scaffolds in fragment-based design. I’ve had colleagues spark fierce debates at poster sessions about its best functionalization routes—proof that innovation hasn’t run dry.
Rats and cell lines have endured their fair share of thiamorpholine dosing in toxicology screens. Results show moderate to low acute toxicity, though respiratory and dermal exposure still brings needs for caution—sulfur and nitrogen heterocycles often find unexpected ways to disrupt metabolic pathways. Chronic exposure data remains sparse, but repeated animal dosing tends to target liver and kidney parameters, prompting regulatory types to push for proper containment and exposure tracking. I remember a colleague recounting an incident after low-level exposure; though symptoms passed quickly, that story stuck around as a constant reminder that benign appearance doesn’t guarantee harmlessness. Academic and industrial toxicologists keep pushing for broader environmental fate studies to spot breakdown products, worried that sulfur-containing rings could show up as unexpected contaminants.
Looking ahead, thiamorpholine’s prospects hinge on bridging the gap between niche research tool and mainstream industrial input. Breakthroughs in process chemistry—cheaper, cleaner synthesis; greener purification; or more accessible precursors—could swing the door open to wider application. Medicinal chemists still chase ring systems that dodge common metabolic pitfalls, and thiamorpholine holds promise as a way to finesse drug profiles against emerging resistance threats. Environmental chemists think its derivatives might serve as building blocks for more robust, less environmentally persistent agrochemicals. Yet its broader adoption will stick on safety, cost, and demonstrable advantages over cousins like morpholine or piperazine. Every year brings sharper analytical tools and fresh curiosity; with enough persistence and imagination, the thiamorpholine story will outgrow its current chapter.
Thiamorpholine has one of those names that only a chemist could love, but plenty is riding on this small, sulfur-nitrogen ring. This compound does not often turn up in daily conversations, yet its presence can ripple through several industries in ways you might not expect. I’ve spent time talking with folks who walk factory floors, and many of them know these substances by function instead of IUPAC labels. So let’s pull thiamorpholine out of the technical shadow and break down where it really matters.
Chemists working with thiamorpholine usually keep their focus on what this compound can build. The sulfur and nitrogen together help create building blocks for larger, more complicated chemicals. One of the bigger roles thiamorpholine plays is in the pharmaceutical industry. Researchers turn to it when developing active ingredients for medications, especially those aiming to treat heart and nervous system problems.
Thiamorpholine often joins the recipe because its ring brings stability and changes the way molecules behave in the body. In making drugs, small shifts matter. Adding a sulfur atom where a carbon used to sit can turn an ordinary substance into a medicine with new potential. Chemists tinker with the structure, swapping out groups or blending thiamorpholine with other small rings until they find a match for a drug that fights the right disease. That creative problem-solving sticks with me—behind every pill sits hours of experimentation with molecules like thiamorpholine.
Pharmaceutical applications aren’t the only place for thiamorpholine. Chemical manufacturers often use this compound as a starting point for industrial solvents and corrosion inhibitors. In refineries, keeping pipes clear is no joke. Thiamorpholine-based products can help ward off rust and mineral build-up, extending the lifetime of expensive equipment. It may not grab headlines, but saving a major pipeline from a shutdown is the kind of win that matters for jobs and costs.
Paints and coatings sometimes lean on thiamorpholine derivatives too. The sulfur-nitrogen blend changes the mix just enough to help paints resist harsh chemicals and last longer outside. People working on production lines pick up on these subtle shifts—using thiamorpholine often means products stand up better to weather, heat, and time.
Any time you deal with chemical building blocks, there’s risk. Thiamorpholine itself isn’t terribly well-known outside industry, but its derivatives can range from helpful to hazardous. Some can irritate skin or eyes, and workers deserve protections if they handle this stuff daily. Safety calls for solid training, good ventilation, and strict handling rules. Looking at data from labs and plants, these commonsense steps cut down on accidents—a practical lesson I’ve seen firsthand.
Environmental impact is another piece of the puzzle. By tracking how waste flows from factories and pushing companies to close loops, cities can keep thiamorpholine and its cousins out of waterways. Regulators and manufacturers who work together here tend to find better results. In some places, recycled solvents and closed systems have dropped emissions and saved money—proof you don’t have to pick between cleaner air and factory jobs.
Thiamorpholine doesn’t get the spotlight, but that’s normal for chemical intermediates. It rarely takes a starring role, but the ripple effect from this compound runs through the medicines people take and the materials that last longer in tough conditions. Engineers, chemists, and plant workers build on each other’s knowledge, turning simple rings into real progress. That’s the kind of teamwork and innovation I keep seeing in every corner where chemistry meets daily life.
Understanding molecules unlocks a new way of looking at the world. Thiamorpholine isn’t a headline-grabber like caffeine or aspirin, but knowing its structure gives a peek into what makes it tick, why it pops up in labs, and how it fits into modern chemistry. Plenty of folks cruise through daily life without giving chemistry a thought. I used to blank out at the sight of hexagons on whiteboards, until I realized that these diagrams hold stories about reactivity, safety, and possible uses.
Thiamorpholine belongs to a group called heterocycles. Think of heterocycles as molecular rings that use a bit of “guest starring”—carbon builds the backbone, but other elements take a seat too. For thiamorpholine, that lineup features both a sulfur and a nitrogen atom, tucked into a six-membered ring. Chemists write its molecular formula as C4H9NS, which means you’ll find four carbons, nine hydrogens, one nitrogen, and a single sulfur.
This ring structure reminds people of morpholine, a similar chemical where the only guest is an oxygen. Here, sulfur replaces oxygen. Small change on paper, but big consequences for reactivity. The sorted-out structure looks like this: a ring where sulfur and nitrogen atoms sit opposite each other, with four carbons filling in the gaps. Sulfur’s in spot one, nitrogen in spot four. Some call this arrangement “1,4-thiazane.” Seen under the microscope, the atoms bend into a puckered ring, not a flat plate. This arrangement helps explain why thiamorpholine behaves a little differently than other compounds from the same family.
If you look at the periodic table, sulfur is heavier and bigger than oxygen. It brings different electrons to the party. In real-world terms, that means thiamorpholine stands up better to strong bases, but breaks down more easily when acids are around. Lab folks actually lean into this for certain syntheses. They might reach for thiamorpholine when they want a ring that can flex more or react under gentler conditions.
From my own experience in a university lab, swaps like this sometimes lead to new results by accident. One swap gave us a compound that acted completely differently in reactions. Subtle changes in the structure—just one atom—can change the whole outcome. Thiamorpholine doesn’t show up in every medicine cabinet, but this small tweak has helped scientists build molecules that fit more snugly into enzymes or interact with metals for catalysts.
One thing about compounds with sulfur and nitrogen: sometimes, sniffing them brings to mind a rotten-egg stench. That depends on how exposed that sulfur sits and whether decomposition starts. Chemistry students often get nervous when asked to handle “stinky ring” compounds. Yet, thiamorpholine has value because it behaves more cleanly than some cousins. Of course, handling any organic ring compound means gloves, goggles, and a well-ventilated hood—nobody wants to gamble with unseen hazards. Waste handling demands care too; sulfur-containing molecules sometimes cause problems in waste streams, so neutralization steps become part of any solid protocol.
Thiamorpholine may not make its way into household products, but scientists keep learning from its behavior. With more focus falling on sustainable processes, research pushes toward safer rings and improved handling methods. Better understanding this molecule’s quirks could provide a springboard for inventing greener reactions or designing specialty drugs. Every atom swapped or twisted reshapes what’s possible.
Thiamorpholine sits in a place I don’t see every day, between lab science and industrial chemistry. Working around chemicals like this taught me respect, patience, and the value of reading the fine print. Thiamorpholine makes itself useful in chemical manufacturing, pharmaceuticals, and even some specialty coatings. But don’t let that clean resume fool you—one splash can turn a smooth day into a trip to the emergency room.
Nobody shows up to a race without a helmet. Same goes for chemical handling. My early nerves around unknown liquids kept me alert. I always grab gloves—nitrile or neoprene, latex falls short with these kinds of substances. Safety goggles or a face shield come next. Chemicals aren’t forgiving if they catch you blinking. Sometimes a long-sleeved lab coat or chemical suit is the only thing between you and a rash that stays longer than a bad idea. Closed shoes and a sturdy pair of pants help, too.
Years back, I learned the hard way that fumes can sneak up on you. Thiamorpholine gives off vapors and the nose can’t always be trusted. Fume hoods need to swallow up every whiff, so I keep work in ventilated spaces only. If extraction isn’t bulletproof, a proper respirator—one rated for organic vapors—sits around my neck. I always help new folks get masks fitted properly. Too many old-timers lost their lungs thinking a cracked window would do the job.
Spills happen. Pretending they don’t is the fastest way to call for an ambulance. I’ve always kept absorbent pads, soda ash, or lime powder within arm’s reach. Easy cleanup beats fighting a spreading mess. After any spill, washing the affected skin with water for at least 15 minutes does more good than panicking. Any splashes to the eyes get treated as an emergency, no hesitation. I’ve watched friends pour out whole gallons of eye-wash solution after a single careless moment. It sounds harsh, but it’s a small price.
I made the mistake of storing reactive compounds on crowded shelves once. Thiamorpholine prefers cool, dry, and well-labeled containers—no leaky lids, no sunlight. Big bold warning signs help, especially for folks who don’t live in the chemical world daily. Locking cabinets or chemical fridges add peace of mind and cut down on accidents, especially if kids or curious staff are around.
I grew up around mechanics and welders who passed down tips like family recipes. Formal training matters just as much. Regular refreshers remind teams to flush out lazy habits. Real safety doesn’t come just from manuals, but from walking the talk and calling out dangerous shortcuts. Emergency plans with clear evacuation routes, contact numbers, and first-aid instructions hang by every sink and door in places I trust.
Improving chemical safety calls for commitment across the board. Investing in better ventilation, modern spill kits, or safer storage pays off over time. A no-questions-asked policy about reporting close calls keeps the whole crew honest and helps us stay ahead of trouble. Listening to people who’ve lived through accidents turns statistics into real warnings.
Mistakes made me careful, stories from others taught me respect, and a healthy dose of skepticism makes handling thiamorpholine possible without fear. Good safety follows you home, and nobody wants to bring an accident through the front door.
Thiamorpholine doesn’t usually pop up in household conversation, but anyone working in a lab or with specialty chemicals probably knows it’s a sulfur-containing heterocycle. It plays a role in organic synthesis and the production of pharmaceuticals and agrochemicals. I’ve seen people stack bottles of this stuff in backroom cabinets and then forget about them for years. That’s risky — with specialty chemicals, losing track can cost time and resources, not to mention the safety concerns.
Bottled thiamorpholine tends to look stable on the shelf. In its pure form, it doesn’t put on a show; it’s colorless, it doesn’t smell strong. But that outward calm can fool you. Typical shelf life lands between one and two years if the seal stays tight and it’s away from heat and light. Once oxidation gets a start, decomposition isn’t far behind. In labs I’ve worked in, people have found old bottles gone yellow after a few seasons sitting near sunlit windows. Once it turns, you’ve lost more than just your investment — you face dangerous byproducts.
For thiamorpholine, good storage isn’t fancy, just careful. Keep it in tightly sealed containers, away from moisture. Dry air matters. Sulfur rings attract water, and humidity leads to hydrolysis. In my own experience, lazy stacking next to water-based reagents never ended well — a little condensation inside a cap and the entire bottle became trash. An opaque container doesn’t hurt, as light can add energy for unwanted reactions. Temperature matters, too; room temp works, but don’t leave it near heaters or in a sunbeam. Cooler basements with consistent airflow beat stuffy closets every time.
Wasting chemicals hits hard, both in small labs and big companies. A big part of my early work was tracking down causes when experiments failed. More often than not, we traced problems to old or improperly stored chemicals. Thiamorpholine sitting past its prime can lose key properties — no reaction, weak results or, worse, unpredictable side-products. Mistakes like these waste more than time: they eat into budgets, force extra orders, and sometimes spark cleanup headaches. I’ve watched researchers nearly scrap whole projects because a half-used bottle turned bad one month before a deadline.
Treat these chemicals like food with an expiration date. Add a clear label with the date opened and keep a log. Build routines for regular checks, especially with tricky or uncommon reagents. Centrifuge tubes with silica gel packs in storage bins often save trouble in damp climates — I’ve gotten into the habit of replacing them every few months. Central chemical inventories help larger groups dodge overlap and accidental neglect. Lab managers can set up reminders or a spreadsheet to flag supplies after one year. Dealing with hazardous waste costs far more than planning ahead.
It’s not glamorous work: inspecting bottles, double-checking seals, keeping spaces organized. But these small habits save bigger headaches. I’ve learned that any hope of long shelf life for thiamorpholine begins with respect for details. Sticking chemical containers in the right spot today prevents expensive problems down the road.
People love to talk about chemicals in abstract ways, but those who have handled thiamorpholine know purity isn’t just a technical note on a data sheet. Walk through a chemical manufacturing plant or work in a research lab, and you quickly realize: not all thiamorpholine comes in the same quality. Purity grades mean something—especially when things start to go sideways.
I’ve seen more than a few small companies hunt for “just the cheapest bottle” of thiamorpholine, but that approach usually bites back. Industrial grade might come at a discount, but look closer and you start to see what you pay for. For pharmaceuticals, the bar sits higher—sometimes over 99% pure. If that purity slips, impurities creep in. Chemistry doesn’t forgive missteps: even fractions of a percent can gum up a reaction, create unwanted by-products, or in the worst-case scenario, spark wild safety issues.
I’ve watched teams spend days chasing their tails, thinking they messed up their synthesis. In the end, it turned out the “cheaper” thiamorpholine had enough contaminants to wreck the whole batch. No one likes telling the boss they wasted a week on a failed run thanks to a bottle that saved them $30.
People sometimes overlook where purity matters most. In agriculture, a lower grade might not always break things—but use that same drum for a new medicine or high-end electronics, and all hell breaks loose. Imagine explaining to regulators why your process choked on an “invisible” impurity. The FDA doesn’t find it funny, and neither do production managers burning money on rejected batches.
I remember a colleague who tried to improvise with leftover industrial-grade supply for a pilot-scale pharmaceutical test. Only after a pile of paperwork, troubleshooting, and a week’s delay did the supplier point out the non-pharma grade had trace amines. Those traces? They led to regulatory headaches and a failed inspection. Small difference on paper, huge pain in practice.
People sometimes forget sourcing isn’t just about getting “thiamorpholine” stamped on the drum. Consistency matters too. During COVID, supply hiccups left teams scrambling. Some looked for new vendors, and a few thought any grade could fill the gap. Not so fast—unexpected results from a less reliable grade can halt production lines and lead to recalls. In this game, reputation can take a major hit from one batch gone wrong.
Sensible teams trust only suppliers with solid reputations. I’ve found it pays to demand a certificate of analysis for every lot—especially for higher stakes labs. Double-checking batch purity up front saves a mountain of trouble later.
Some companies invest in their own routine checks—gas chromatography, NMR, or even a simple melting point comparison. It’s not paranoia; it’s damage control. Cutting corners on purity isn’t just about the immediate reaction; it’s about what happens downstream. Cleaning up a preventable mess costs more than paying for a high-purity grade at the beginning.
So yes, thiamorpholine comes in different grades. The next time someone shrugs it off, ask who wants to take responsibility for a contaminated production run. That question tends to make the penny pinchers think twice.
| Names | |
| Preferred IUPAC name | 1,4-Thiazinane |
| Other names |
4-Thiamorpholine Tetrahydro-1,4-thiazine Thiamorpholine, 1,1-dioxide |
| Pronunciation | /ˌθaɪ.əˈmɔː.fəˌliːn/ |
| Identifiers | |
| CAS Number | 123-95-5 |
| Beilstein Reference | 1208961 |
| ChEBI | CHEBI:53264 |
| ChEMBL | CHEMBL477262 |
| ChemSpider | 54765 |
| DrugBank | DB01591 |
| ECHA InfoCard | 100.032.208 |
| EC Number | 220-949-2 |
| Gmelin Reference | 158501 |
| KEGG | C18594 |
| MeSH | D017962 |
| PubChem CID | 123148 |
| RTECS number | KY9650000 |
| UNII | MVU5K76F6A |
| UN number | NA1993 |
| CompTox Dashboard (EPA) | DTXSID8020223 |
| Properties | |
| Chemical formula | C4H9NOS |
| Molar mass | 133.21 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Odorless |
| Density | 1.17 g/mL at 25 °C |
| Solubility in water | Soluble |
| log P | -0.44 |
| Vapor pressure | 1.5 mmHg (25°C) |
| Acidity (pKa) | 8.6 |
| Basicity (pKb) | 5.81 |
| Magnetic susceptibility (χ) | -62.8·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.506 |
| Viscosity | Viscosity: 1.36 cP (20°C) |
| Dipole moment | 3.56 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 322.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -3921.7 kJ/mol |
| Pharmacology | |
| ATC code | N05CC03 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and serious eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05 |
| Signal word | Warning |
| Hazard statements | H302+H312+H332: Harmful if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | P264, P270, P273, P280, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 82°C |
| Autoignition temperature | 215°C |
| Explosive limits | Explosive limits: 4.1–20.8% |
| Lethal dose or concentration | LD50 (oral, rat): 188 mg/kg |
| LD50 (median dose) | LD50: 56 mg/kg (intravenous, mouse) |
| NIOSH | THA80410N9 |
| PEL (Permissible) | PEL for Thiamorpholine: Not established |
| REL (Recommended) | 2-[(Methylthio)methyl]morpholine |
| Related compounds | |
| Related compounds |
Morpholine Thiomorpholine Piperazine 1,4-Oxazepane 1,4-Thiazepane |