Scientists first explored the chemistry of tetrahydrothiophene derivatives in the early half of the twentieth century. As industry turned away from hazardous and unstable reagents, sulfone compounds like tetrahydrothiophene 1,1-dioxide grabbed attention, specifically as stable alternatives in organic synthesis. Early literature highlights both fascination and skepticism—some researchers doubted practical uses outside pure academic circles, others pointed to its promise in manufacturing and synthesis. Over the decades, the compound proved its worth in pharmaceuticals and polymer science, as researchers sought ways to manipulate sulfur-oxygen chemistry for broader application. Tetrahydrothiophene 1,1-dioxide's journey from obscure curiosity to staple laboratory reagent tracks with a larger shift in chemical industry: minimizing risk, maximizing useful function.
Tetrahydrothiophene 1,1-dioxide, commonly known as sulfolane, appears as a clear, colorless liquid with a mild odor. Its widespread adoption in industry largely comes from its polar aprotic solvent character, which enables streamlined recovery and reuse in processes ranging from gas purification to electrochemical applications. Chemical catalogs list the compound under a few synonyms: sulfolane, thiolane-1,1-dioxide, and 2,3,4,5-tetrahydrothiophene 1,1-dioxide, to name a few. Unlike some niche reagents, large-scale manufacturers have standardized its specifications, making it available in purity grades that suit both research benches and industrial reactors.
Standard physical data puts tetrahydrothiophene 1,1-dioxide’s boiling point near 285°C with a melting point around 27°C. Its relatively high dielectric constant makes it a favorite for dissolving both organic and inorganic salts in chemical synthesis. Water miscibility adds another positive in many process flows, eliminating the need for troublesome co-solvents. Chemically, this sulfone stands apart for its resistance to both acids and bases under ordinary conditions. The molecule’s stability under heat and pressure supports its use in demanding extraction protocols, where lesser solvents would decompose. Some industries value sulfolane’s neutrality—meaning it rarely reacts with reagents unless pushed hard by a catalyst or extreme conditions.
Reliable product labeling tends to include the CAS number 126-33-0, along with batch purity—most reputable vendors promise at least 99% assay. Manufacturers usually indicate moisture content, residual acidity, and refractive index, all essential for customers who rely on tight process tolerances. Packaging commonly comes in galvanized drums or fluoropolymer-lined containers, as contact with reactive metals can introduce contaminants that interfere with sensitive reactions. Shipping guidelines fall under controlled substances only for specific end uses, but general handling follows chemical safety standards familiar to most laboratory workers.
Most commercial synthesis routes begin with tetrahydrothiophene, which oxidizes using hydrogen peroxide in the presence of suitable catalysts. Some routes exploit sodium metaperiodate, but for bulk production, peracid oxidations tend to be more cost-effective and scalable. Control of temperature, reaction time, and stirring ensures high yield and purity. Laboratories experimenting with small batches often favor milder oxidations, since the economics of scale don’t outweigh the benefit of gentler conditions. What stands out here is the relative simplicity—no rare metals, no elaborate pre-functionalization steps, just tried-and-true sulfur oxidation chemistry.
Tetrahydrothiophene 1,1-dioxide participates in a select group of chemical reactions. Its chief contribution comes from its role as an inert polar medium, especially for SN2 displacement reactions where typical solvents like DMF or DMSO may introduce unwanted byproducts. While the core sulfolane structure resists most transformations, it does show some reactivity at the 2- and 5-positions, especially under strong base or with transition metal catalysis. Electrochemical studies suggest the potential for ring-opening or radical-based transformations, which currently remain mostly in the research phase. Rarely does it act as a nucleophile or electrophile under ambient conditions, setting it apart from more reactive sulfur-based molecules.
Beyond the commonly accepted ‘sulfolane,’ chemical catalogs and patent literature also mention alternate spellings like thiolane dioxide and 1,1-dioxo-tetrahydrothiophene. Some regional suppliers label it by process grade, such as “extractive distillation sulfolane” or “ultra-pure sulfolane,” reflecting intended industrial use. Regardless of naming, regulatory papers reference its CAS number for clarity and traceability, especially in safety documentation.
No modern commentary feels complete without attention to health and operational risk. Handling sulfolane demands the same care given to any polar organic solvent: gloves, goggles, and plenty of ventilation. Inhalation exposure should stay minimal; while acute toxicity remains low, chronic effects from high concentrations lack comprehensive study. Environmental releases deserve mitigation—sulfolane lingers in groundwater, with detected contamination in some industrial regions sparking regulatory reviews. Standard operational protocols limit sources of ignition and emphasize routine checks for container durability, especially given the material’s capacity to leach through less-resistant polymers. Spills call for immediate containment and collection, with proper waste processing to avoid bioaccumulation.
The biggest market for sulfolane sits in natural gas processing, where it scrubs aromatic hydrocarbons and sulfur compounds from fuel stocks with efficiency hard to match. Petrochemical plants rely on its stability and solvent power to cleanly separate critical feedstocks, especially during extractive distillation. Electrochemical industries look to sulfolane as an electrolyte fluid, making use of its high dielectric constant. Some pharmaceutical routes leverage sulfolane’s polar environment to favor difficult substitution reactions or to solubilize challenging intermediates. Battery research uses sulfolane for specialty electrolytes, seeking to balance flammability and conductivity. Each year, new patents describe both tweaks to these formulas and surprising off-label uses, from polymer synthesis to the fine-tuning of specialty adhesives.
Current laboratory attention focuses on greener synthesis routes and tighter purification to trim environmental impact. Some teams track down sustainable oxidants that cut down on waste and energy use during sulfolane production. Others pursue functionalized derivatives, aiming to combine sulfolane’s outstanding solvent abilities with specific catalyst compatibility or targeted biological activity. Universities and chemical companies alike report experimental uses as a reaction medium for transition metal catalysis, ionic liquid formation, or in high-voltage battery systems. The thrust of development turns on increasing both safety and versatility, bringing this once-overlooked sulfone into modern synthetic chemistry’s main stage.
Sulfolane’s toxicity story involves both documented acute effects and growing concerns about chronic exposure. Animal studies point to relatively high LD50 doses, indicating low risk during routine laboratory use. Even so, field reports from industrial sites detail groundwater contamination, raising alarms in regions where prolonged exposure could reach wildlife or drinking sources. Long-term studies examine potential liver and kidney effects, but consensus remains elusive given the breadth of environmental data needed. Regulatory agencies insist on both occupational exposure limits and ongoing environmental monitoring. Chemical engineers respond with investment in robust containment, monitoring technology, and next-generation remediation practices. Safer process design and closed-system handling count as the best frontline defenses until research reveals more about chronic effects.
Sulfolane’s future rides on society’s push for cleaner industrial chemistry. Market demand directs research funds toward biodegradable or less persistent alternatives, yet technical teams keep uncovering new tricks with this sulfone. Enhanced process control and better analytical tools promise to cut losses and curb accidents. Cross-disciplinary projects team up with toxicologists to map out risk much more clearly, giving community health and industry smoother roads ahead. As chemical manufacturers move toward circular resource use—reusing solvents, reducing hazardous waste—sulfolane stands to benefit from both regulatory shifts and scientific curiosity. The compound’s history of resilience, combined with ongoing adjustments, points toward an industrial role shaped not just by tradition, but by need-driven innovation.
Tetrahydrothiophene 1,1-dioxide doesn’t show up in daily headlines or get chatter at cocktail parties. In the world of chemistry, though, it pulls more weight than many realize. This compound stands out for its role as a solvent and as a building block in making other chemicals. Chemists and industry veterans have dealt with its unique smell and properties for decades. Its real value often comes from how it supports bigger processes, not as some superstar ingredient.
You’ll find tetrahydrothiophene 1,1-dioxide, known in many labs as sulfolane, working behind the scenes in refining oil and processing natural gas. Refineries use sulfolane because it dissolves non-polar and polar compounds really well. Companies rely on this property to clean up certain fuels, pulling out impurities like aromatics that would otherwise lower fuel quality. Getting clean fuel out of raw oil turns out to be a messy job. Without solvents like this one, a lot of the gasoline and jet fuel in circulation wouldn’t meet the standards we expect.
The compound also pops up in the field of pharmaceuticals. Every time I hear about a new drug being designed, I picture the quiet, colorless liquid helping dissolve stubborn chemicals or supporting a reaction that would stall without it. Chemists favor sulfolane because it doesn’t break down easily, even under some tough conditions involving strong acids or high temperatures.
Anyone who’s spent time in a refinery or chemical plant can tell you: safety with chemical solvents requires vigilance. Tetrahydrothiophene 1,1-dioxide can irritate skin and eyes, and it shouldn’t build up in the environment unchecked. A few years back, I worked on a site where we reviewed groundwater samples for traces of sulfolane, and the concern wasn’t far-fetched. It dissolves in water, so improper handling or leaks mean it can move through soil more quickly than heavier compounds.
Experts have flagged groundwater contamination in some parts of the world, and this prompted regulatory agencies to call for closer tracking of emissions and disposal. These are lessons learned the hard way. Anyone storing, transporting, or using sulfolane must keep equipment tight, monitor for leaks, and update cleanup measures. The push for stricter standards came not from distant research, but from stories of wells and aquifers affected near industrial facilities.
Chemical engineers keep searching for alternatives that do the job of tetrahydrothiophene 1,1-dioxide without the same risks. Some new solvents break down faster if spilled, and more companies are building equipment designed to recover and recycle sulfolane, shrinking both costs and environmental footprints. I’ve seen pilot projects in the works that capture these solvents and purify them onsite for another round in the process.
Research keeps pushing the boundaries, looking for ways to get the same cleaning power from safer, greener molecules. Until a truly safe drop-in emerges, managing risk around established solvents like this one remains a daily priority. People in the industry stay sharp because the safety routines and monitoring systems adopted today keep accidents and exposures from growing into tomorrow’s headline.
Tetrahydrothiophene 1,1-dioxide, also called sulfolane, doesn’t get much limelight outside of chemical engineering or production labs. Let’s give some attention to its structure—it makes all the difference. You’re looking at a five-membered ring: four carbon atoms, one sulfur atom. The “1,1-dioxide” part means this sulfur gets double-bonded to two oxygens. Chemically, you see a saturated ring, no double bonds between carbons, and the sulfur in the middle forms two sulfone (SO2) groups sticking off. To write it out, the structure is C4H8SO2.
That sulfone group changes everything. It’s what gives sulfolane strong polarity. A molecule with oxygen double bonds like that tugs on electrons—so it loves water and readily dissolves all sorts of polar and nonpolar stuff. This structure pushes sulfolane into serious workhorse territory for folks separating chemicals, cleaning up natural gas, and working on purification. Years back, I watched a technical crew struggle to extract aromatic hydrocarbons efficiently. Switching the solvent to sulfolane cut through the problem, simply because of the way those electronegative oxygens open up possibilities that you don’t get with plain hydrocarbons or sloppy mixtures.
It goes beyond trivia. Plenty of chemicals look almost identical on paper but act differently because a single atom or group moves position; sulfolane’s ring shape and those distinctive sulfone groups change its behavior. This matters for safety—strong polarity leads to lower volatility than you might expect compared to its “family” members in organic chemistry. If you’re stuck managing dangerous vapors in a plant setting, low volatility and high boiling points mean fewer headaches around air quality rules and worker safety.
Digging through case studies and reviews, you realize why regulatory minds care so much about the chemical shape. That sulfone structure stays stable in water, doesn’t break down quickly, and can build up in the environment. I’ve seen folks get caught off-guard when they handle sulfolane like it’s just another solvent. It persists—so you can’t treat it like fast-degrading alternatives. The sulfone ring, double-bonded oxygens, and saturated carbons mean it resists breakdown unless you really push it in a treatment setup. This shifts the way you think about wastewater disposal and spill response. Places in Alaska have faced real headaches from groundwater contamination when operators underestimated that persistence.
Don’t ignore the nitty-gritty details of molecular shape. Strong research points to the need for technologies that break down sulfolane after it’s done its job. Oxidative treatments or specific biodegradation approaches can chop apart that tough ring, but each site might need a custom solution. Process engineers would do well to keep a close relationship between chemical knowledge and site safety—designing operations around the quirks of compounds like sulfolane. If you’re in the field of remediation, knowing the structure means knowing which catalysts or microbes to apply.
No one builds a safe process on half-knowledge. There’s real value in getting familiar with the twists and turns of molecules like tetrahydrothiophene 1,1-dioxide. Whether you’re extracting aromatics, keeping the air clean, or fixing groundwater, a detailed mental picture of the ring and its double-bonded oxygens can only help. History in industrial chemistry keeps reminding us—understanding the structure does more than tick a box; it shapes results and limits risk.
Tetrahydrothiophene 1,1-dioxide, often referred to as sulfolane, pops up in settings like oil refining, specialty chemical manufacturing, and solvent applications. This isn’t a household name for most people. I first came across it in a laboratory safety meeting at a research facility. Right away, the focus landed on safe handling, personal protective equipment, and storage protocols. Folks working with it understood that a chemical doesn’t need to be explosive or corrosive to spark health concerns.
This colorless liquid carries some interesting capabilities in industrial processes. That’s why it ends up on chemical inventories worldwide. Still, safety data sheets often highlight risks that can’t be ignored simply because injuries rarely make headlines.
According to research from regulatory agencies and workplace health organizations, sulfolane’s acute toxicity sits in the moderate range. My experience matches up with the data—exposure through vapor or skin contact creeps up slowly, showing effects like headaches, dizziness, or mild respiratory irritation. Prolonged or repeated contact with skin sometimes leads to rash or dryness. Inhaling high concentrations tends to irritate the throat and lungs.
Scientists have run animal studies with sulfolane showing potential effects on the nervous system, liver, and blood. The U.S. Environmental Protection Agency and the Agency for Toxic Substances and Disease Registry both caution workers and communities near large-scale manufacturing to monitor for exposure, especially through drinking water. Groundwater contamination has turned up near chemical facilities before. The Canadian government, for example, flagged sulfolane contamination nearly two decades ago and investigated community health after it made its way into wells.
Ignoring these risks doesn’t serve workers, local residents, or consumers. People in the chemical industry deserve straightforward access to health and exposure information. I’ve watched companies prioritize job-site safety investments when risks stem from both headline-grabbing hazards and more subtle, long-term chemical threats. Insurance companies and worker unions push for better monitoring as well.
Because sulfolane is persistent and doesn’t break down easily underground, spill clean-up pulls in environmental engineers for years. Costly remediation can follow. Regulatory trends suggest that more jurisdictions expect plants to install advanced containment and waste management. These rules not only protect water sources but also reinforce the need for active and transparent communication across supply chains.
I’ve found that practical risk mitigation means wearing the right gloves, keeping chemicals away from food and drink, and making sure spill response tools stand ready. Supervisors should share regular training and updates when new research on solvents like sulfolane appears. For companies near neighborhoods or rivers, close coordination with local water authorities becomes essential. Continuous monitoring helps spot leaks fast and avoids large-scale contamination.
Switching to greener alternatives doesn’t always prove simple, but innovation in green chemistry offers new options for certain applications. In the lab, we trialed replacement solvents when evidence showed consistent health concerns. Community feedback matters. If residents near factories raise questions about water quality, quick and honest responses build trust and keep people safer.
Every chemical brings its own set of quirks, and Tetrahydrothiophene 1,1-Dioxide falls squarely into the “handle with respect” category. In my time working around specialty labs, I’ve watched smart and seasoned scientists scramble when an “innocent” sample seeped through a compromised container. This isn’t some inert powder. Tetrahydrothiophene 1,1-Dioxide can bite back with skin and respiratory irritation, and once a spill gets going, cleaning it up without proper know-how ends up wasting gloves, wipes, and precious peace of mind.
Storing this compound calls for real vigilance—much like locking up strong acids or reactive agents. You want a cool, dry spot. High humidity or a warm storeroom can send quality downhill fast, with moisture kicking off decomposition or product caking that turns precise dosing into a guessing game. Every time I see someone shove an ill-sealed jar onto a random shelf, I cringe. Sealing freshness means airtight containers—glass or chemical-resistant plastic—snap shut, labeled clearly with hazard warnings.
Ventilation plays a major part in safety, too. I’ve walked into store rooms after a summer break to the sharp sting of chemical odor. Proper ventilation strips volatile fumes from the room air, keeping concentration low and preventing that slow, toxic buildup. Simple shelving works only if ventilated. Too many storerooms close up tight to save on HVAC, but one leaky bottle can ruin the air for everyone.
No one wants a trip to the eyewash station, but plenty of new techs try to take shortcuts on goggles or gloves. Rubber or nitrile gloves, chemical splash goggles, and a lab coat mean far less chance of getting burned or breathing in a vapor. My own close calls came from thinking “it’ll be quick, I’ll just pour it in,” and catching a faceful of unexpected odor. The rules aren’t just overkill—they’re hard-won by those who’ve gotten burned, sometimes literally.
Handling Tetrahydrothiophene 1,1-Dioxide works out best with clear procedures. Pouring or transferring always happens inside a fume hood—never out on an open bench. A spill outside proper containment can go from manageable to mayhem in minutes. Absorbent material rated for chemical spills and an emergency shower in reach sound excessive to some, but I’ve seen both save a lot of pain.
Disposal won’t win any awards for glamorous work, but cutting corners comes back to bite hard. This isn’t something anyone flushes or throws out with the regular trash. Designated waste containers—labeled for hazardous organic materials—keep dangerous leftovers out of drains and off landfill. Local environmental health rules vary, but flouting them brings steep fines and more serious fallout if cleanup crews get involved.
By respecting every bottle, every storage call, everyone at the bench steps into a safer space. Shared knowledge, clear labeling, and strict PPE routines protected my teams more often than luck ever did. In labs and storerooms, good habits mean more than quick shortcuts—especially with chemicals like Tetrahydrothiophene 1,1-Dioxide in the mix.
Anyone buying chemical products wants clarity about purity. I’ve seen people overlook this detail, only to realize later that a high purity standard makes a real difference—whether it’s for research, manufacturing, or health-related applications. Purity reflects how much of the product truly is what the label says. Impurities, even tiny ones, can interfere with experiments, cause unexpected side effects, or impact how a final product performs.
Laboratories often list purity as a percentage, such as 98%, 99%, or even higher for pharmaceutical needs. These values aren’t decorative. They signal the level of unwanted substances remaining after production. For instance, pharmaceutical companies can’t cut corners here—the FDA and equivalent agencies keep a close eye on these numbers because a minor contaminant might harm someone or render a medication useless.
Beyond labs, I’ve spoken with manufacturers who stress that high-purity chemicals limit batch-to-batch variation. In rare cases, lower grades may work for less sensitive uses, like cleaning large industrial parts. Still, for food and medical purposes, trace chemicals might become dangerous, so sticking to trusted suppliers who freely provide purity documentation really matters. Never hesitate to ask for a certificate of analysis. Responsible sellers supply it as proof.
Packaging shapes how useful and safe the product becomes. I used to think packaging was just about convenience or branding. After talking with warehouse managers, it’s clear that right packaging prevents contamination, moisture exposure, and sometimes even legal headaches.
Bulk buyers often request drums—these can range from 25 kilograms to 200 liters, depending on the product’s stability and shipping regulations. Solid forms sometimes ship in polypropylene bags with double layers; liquids need chemical-resistant barrels or small HDPE bottles if high precision is necessary. In my experience, working with smaller labs, I’ve seen a growing demand for 500-gram or 1-kilogram packages. People don’t want leftovers sitting around longer than needed, and smaller packages reduce waste, especially with sensitive or reactive materials.
Some suppliers offer custom pack sizes. For research projects, this saves cost and reduces handling risk. A reliable supplier never hesitates to discuss packaging solutions before a deal closes. In regulated industries, tamper-evident packaging builds trust—one broken seal is enough to make buyers doubt an entire shipment.
Buyers want crystal-clear answers about purity and packaging. Surprises destroy trust faster than anything. I’ve learned from hard mistakes that a vendor should always put details front and center—certificate numbers, shelf life, specific storage guidance, and full disclosure about packaging choices. Transparency isn’t just corporate lingo; it shields people from costly errors and health risks.
Going forward, the best solutions bring producers, suppliers, and end-users together to agree on clear standards for purity and packaging. I’ve seen success when suppliers listen closely to the needs of labs and manufacturers, tailoring options rather than pushing default pack sizes. The result is safer, more efficient work and good long-term relationships between buyers and sellers.
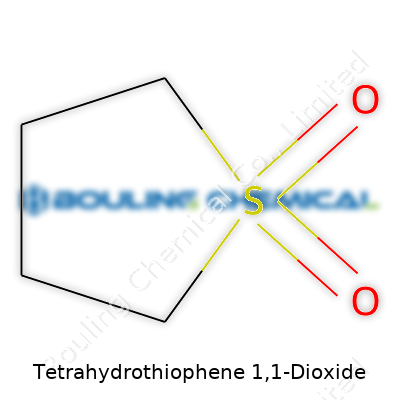
| Names | |
| Preferred IUPAC name | 1λ⁶-Thiane 1,1-dione |
| Other names |
Sulfolane Tetramethylene sulfone |
| Pronunciation | /ˌtɛtrəˌhaɪdroʊˈθaɪoʊˌθiːn ˌwʌn wʌn ˈdaɪˌɒksaɪd/ |
| Identifiers | |
| CAS Number | 110-30-5 |
| 3D model (JSmol) | `3D model (JSmol)` string for **Tetrahydrothiophene 1,1-dioxide**: ``` CS(=O)(=O)CC1CC1 ``` |
| Beilstein Reference | 120755 |
| ChEBI | CHEBI:131320 |
| ChEMBL | CHEMBL50274 |
| ChemSpider | 16255 |
| DrugBank | DB02122 |
| ECHA InfoCard | 100.003.763 |
| EC Number | 209-127-8 |
| Gmelin Reference | 80152 |
| KEGG | C02525 |
| MeSH | D016302 |
| PubChem CID | 7926 |
| RTECS number | WN5250000 |
| UNII | 5B8T83900P |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C4H8O2S |
| Molar mass | 134.19 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.27 g/cm³ |
| Solubility in water | soluble |
| log P | -1.13 |
| Vapor pressure | 0.15 mmHg (25°C) |
| Acidity (pKa) | 15.7 |
| Basicity (pKb) | 2.69 |
| Magnetic susceptibility (χ) | -36.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.487 |
| Viscosity | 17 cP (20 °C) |
| Dipole moment | 4.67 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 203.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -413.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2061.0 kJ/mol |
| Pharmacology | |
| ATC code | N02AX11 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H312 + H332: Harmful if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0-W |
| Flash point | 113°C |
| Autoignition temperature | 330 °C (626 °F; 603 K) |
| Explosive limits | Explosive limits: 2.7–18% |
| Lethal dose or concentration | LD50 Oral Rat 1900 mg/kg |
| LD50 (median dose) | LD50 (median dose): 300 mg/kg (oral, rat) |
| NIOSH | KW2625000 |
| PEL (Permissible) | PEL: 10 ppm (50 mg/m3) (as TWA) |
| REL (Recommended) | 100 mg/m³ |
| Related compounds | |
| Related compounds |
Thiophene Tetrahydrothiophene Sulfolene Sulfolane |