Succinimide entered the chemistry world in the late 19th century, first synthesized as researchers explored derivatives of succinic acid. Early work focused on harnessing the potential of various heterocyclic compounds, with succinimide showing promise in both academic and industrial labs. Its clear structure and reactivity attracted pioneers in pharmaceuticals and organic chemistry. As the years passed, chemists realized that modifying the succinimide ring led to valuable properties, particularly in the anticonvulsant field. This compound formed the backbone for important drugs tackling epilepsy, especially during a time when effective treatment options were scarce. It’s hard to ignore the impact that such a molecule has had on daily medical practice over the decades, especially considering that it originally appeared as a lab curiosity.
Chemists recognize succinimide as a simple, stable, white crystalline solid. Its formula, C4H5NO2, might seem straightforward, but its versatility stretches across numerous industries. In labs, manufacturers sell succinimide under various grades, ranging from technical to pharmaceutical purity. The versatility comes both from its physical properties and from how reliably it behaves in different reactions. For most users, it’s not just a raw material or “intermediate”—it serves as a foundation in protocols ranging from peptide synthesis to electronics cleaning.
A glance at succinimide’s physical data reveals a melting point around 123–125°C, which sits comfortably for handling during synthesis or modifications. Its solubility in water is modest, but it dissolves more easily in polar organic solvents. The molecule itself contains a five-membered ring with two adjacent carbonyl groups, giving it a planar structure that resists hydrolysis under neutral pH but reacts under either acidic or basic conditions. This balance matters because too much reactivity would render it useless in delicate syntheses, while too little would make it hard to modify. Chemists benefit from its neutral odor, low volatility, and minimal hygroscopic tendency, all of which aid in storage and shipping.
Labels on succinimide packaging often highlight assay purity—almost always above 99% for research grade. Users want to see moisture content specified, with typical limits under 0.2%. Impurity profiles include tests for chlorides, sulfates, and heavy metals, ensuring compliance with pharmacopoeial standards for sensitive applications. Common sizes run from small reagent bottles for bench work up through 25-kg drums for industrial operations. Since the substance can irritate skin and mucous membranes, hazard statements warn of necessary handling precautions, including the use of gloves and protective eyewear. Clear labeling helps users manage risk and maintain traceability of the supply chain, especially given international regulations on controlled substances.
In factories and labs, succinimide production starts with the heating of succinic acid or its anhydride with concentrated ammonia or urea. The ammonolysis reaction proceeds under controlled temperatures around 180–200°C, generating ammonia gas and driving the formation of the cyclic imide. Operators monitor the exothermic step and follow with purification by crystallization from hot water, followed by filtration and drying. Some routes use catalysts or specific pressure controls, but even small-scale syntheses echo these straightforward steps. Cost efficiency and yield maximization motivate continuous optimization of temperature profiles, ammonia delivery, and purification workflows. As energy concerns and green chemistry gain traction, some research efforts look to milder or solvent-free synthesis paths, but the classic high-temperature route still dominates commercial production.
Few organic molecules compete with succinimide’s combinatorial possibilities. Its ring opens readily under the right nucleophilic or electrophilic conditions. Chemists exploit this behavior to attach functional groups, synthesize derivatives like N-hydroxysuccinimide (a staple for peptide coupling), or generate more complex imides and cyclic anhydrides. Halogenation leads to compounds with unique luminescent properties, suited for photochemical labels and probes. Reduction delivers flexible building blocks for specialty polymers or agrochemicals. Even the pharmaceutical world finds value in the ease with which succinimide derivatives can fine-tune drug absorption, metabolism, or interaction profiles. This reactivity both opens doors and demands respect: controlling side products, byproducts, and purity takes real skill at the bench.
Depending on context, researchers, suppliers, and regulators may refer to succinimide by several synonyms. Common names include 2,5-pyrrolidinedione and butanimide, though the simplest name tends to stick in conversation. Certain product literature highlights it as amber acid imide, consistent with the connection to succinic acid. Specialty catalogs sometimes bundle succinimide with its family members—like N-methyl or N-hydroxy analogues—under broader “imide” listings. For legal and safety tracking, standard identifiers such as CAS 123-56-8 anchor the documentation, ensuring users find the right compound no matter where their supply comes from.
Fortunately, succinimide carries a manageable safety profile when professionals respect its guidelines. Inhalation or skin exposure can cause irritation, so basic lab and plant safety practices—ventilated work spaces, gloves, eyewear—get the job done. In large-scale facilities, dust control keeps explosions and respiratory risks at bay. Disposal follows established routes for organic solids, generally involving incineration or chemical neutralization. Users reference Safety Data Sheets (SDS) before opening a pack, confirming compatibility with co-stored chemicals, especially oxidizers or strong acids. Regulations require correct hazard pictograms and a well-understood emergency protocol in case of accidental exposure. Ongoing training and safety audits, reinforced by decades of accident data, underpin a mature, well-governed operational culture.
Doctors, engineers, and researchers have each found vital roles for succinimide and its derivatives. In medicine, it forms the core of classic anticonvulsants such as ethosuximide and methsuximide, often prescribed for childhood absence epilepsy. The mechanism draws on its interaction with neural calcium channels, leading to fewer interruptions in daily life for patients. Analytical chemists harness its ability to stabilize or label biomolecules, particularly peptides, through activated intermediates like NHS esters. In electroplating, its stable ring and moderate solubility keep tin and lead surfaces bright and smooth, crucial for electronics manufacturing. Specialty polymers and resins benefit from succinimide’s rigidity and resistance to hydrolysis, expanding material options for advanced coatings or composites. Its presence threads through everything from chromatographic supports to high-performance fuel cell membranes. Every user group leans on those core properties of stability and controlled reactivity.
Universities and R&D centers keep pushing succinimide chemistry into new territory. Current projects explore its derivatives as scaffolds for targeted cancer therapies, exploiting how easily its backbone can be modified to deliver drugs with pinpoint accuracy. Environmental testing labs look to tagged succinimide molecules for trace contaminant detection—its predictable reactivity boosts sensitivity and reliability. Material scientists experiment with linking succinimide rings into polymer chains, aiming for novel electronic, optical, or mechanical properties. With advances in computational chemistry, researchers can now predict how subtle tweaks in the ring can change binding or breakdown rates, leading to a new generation of designer imides. Every new application builds on decades of basic chemistry, while ongoing funding from both public and private sectors signals continued growth.
Work on succinimide’s toxicity spans animal and cell studies. Acute effects tend to be mild by industrial standards, with straightforward irritancy if inhaled or handled without gloves. Chronic exposure, particularly in unprotected workplaces, can cause headaches and skin dryness. Pharmaceutical derivatives bring their own side effects, ranging from mild gastrointestinal upset to rare allergic reactions, which clinicians monitor closely. Regulatory review boards track environmental persistence—fortunately, succinimide does not rank as a major pollutant, thanks to rapid biodegradation and lack of bioaccumulation. Monitoring continues, especially as new analogues hit the market and as advances in toxicological science allow researchers to detect even subtle effects on human health.
Looking ahead, succinimide is set for a broader and deeper role in science and technology. In medicine, new derivatives could target diseases far beyond epilepsy, especially as researchers design drug delivery platforms that harness its modular structure. The growing demand for “green chemistry” and sustainability motivates work on renewable synthesis pathways and more efficient recycling, letting companies cut costs while meeting stricter regulations. The rise of precision medicine and personalized diagnostics leans on the reliability of succinimide-based reagents and detectors. Electronic materials, ever on the hunt for performance and durability, benefit from imide polymers whose properties adjust at the touch of a synthetic chemist. As new tools—automation, AI-driven discovery, and greener manufacturing—change the landscape, succinimide offers a steady foundation, its chemistry as accessible as it is adaptable.
Most folks walk past a chemistry lab and never think about the powders and crystals inside. Succinimide is one of those chemicals scientists reach for in labs and factories, but it never grabs the spotlight like everyday medicines or famous plastics. Yet, if you take apart many essential products, you’ll find succinimide playing an important role.
Pharmaceuticals lean on more than the active drugs in a pill bottle. Take anticonvulsants as an example—medicines that help control seizures in epilepsy. Ethosuximide, one of the main drugs for absence seizures, comes from succinimide. It’s shaped by science to calm overactive brain cells. Without succinimide as a building block, many options for treating epilepsy would shrink.
Drug development is not all about inventing a new molecule. Chemists dig through older ingredients to find ones they can tweak and test safely. Succinimide's basic structure lets chemists build new drug versions, aiming for fewer side effects or longer-lasting results. As epilepsy affects millions, any improvement matters a lot—especially for families dealing with tough cases.
Industries look for chemicals that help their products run longer and faster. Succinimide stabilizes silver in photographic processing, so the photos come out right. This might sound dusty, but film photography still shows up in art, science, and some law enforcement work.
Electronics makers use succinimide compounds for soldering—the process that ties microchips and wires together. Unstable solder can ruin a tiny phone or a big server. Succinimide helps polish and clean metals, making the connections stronger. As gadgets get smaller and faster, this kind of reliability means more devices last longer and land fewer times in the trash.
Rubber manufacturers have another reason to use succinimide. By keeping rubber soft, strong, and protected from aging, succinimide helps produce reliable seals, hoses, and tires. If worn-down parts crack or split, drivers and machine operators pay the price. The public rarely hears what keeps elevators running smoothly or car engines working until winter, but chemistry like this works every day.
As with many chemicals, safe handling matters. While succinimide by itself is not known for high toxicity, factories and labs stay alert to dust, spills, and accidental contact. Workers depend on training and equipment to keep risks low. It helps when companies swap outdated equipment, upgrade safety plans, and keep communication open. Federal regulators, like OSHA and the EPA, update safety limits as studies uncover more details about routes of exposure or long-term effects.
Side effects in medicines always show up sooner or later. Patients with kidney problems or those who take several drugs together must talk honestly with their medical team. Sometimes an ingredient that works great for most does not fit everyone’s body. The FDA tracks these cases and updates advice for prescribers.
It’s tempting to only notice big discoveries and ignore every working part behind them. Succinimide is one of many under-the-radar chemicals that keep modern medicine, technology, and manufacturing ticking over. A small tweak in a formula, or a new warning about side effects, can ripple through products and affect real lives. Careful use, close checks for safety, and a focus on steady improvement turn these quiet helpers into workhorses we rely on—whether we realize it or not.
Succinimide shows up in plenty of places. Chemists know it as a small, ring-shaped molecule. Drug companies rely on it to create certain anticonvulsants like ethosuximide, which helps manage epilepsy. Some food and cosmetics manufacturers also use it in their formulas. Seeing a technical name like this on an ingredient list can make anyone pause and wonder: Is it really okay to use things that sound like they come straight from a lab?
I grew up around folks who read ingredient labels as if they were novels, slow and suspicious. My grandmother wouldn’t let me have a chewing gum until she’d scrutinized each chemical name on the wrapper. Research gives some peace of mind in this case. For medicine, succinimide salts—including ethosuximide and phenytoin derivatives—get prescribed for years without evidence of cancer risk or buildup in the body when used as directed by a healthcare professional. The U.S. Food and Drug Administration approved ethosuximide in the 1960s and continues to monitor its side effects. Most risks relate to misuse or overdose, not everyday doses.
Lab safety data offers another layer. The Environmental Protection Agency and the EU’s chemicals registry classify succinimide as generally safe at low exposure levels. Most reactions come from workers handling concentrated powder—think sheet irritation or mild allergic response—none of which a person would deal with by eating a snack or using a face cream.
Succinimide gets included in medicines and consumer products because, over decades, few people had serious problems after regular use. If someone feels ill after taking a medication containing succinimide, doctors check for allergies or unusual responses, not a built-up threat from the chemical itself. Safety data for food or cosmetics is even less dramatic; regulatory bodies place strict limits on concentrations. The amount sitting in a drug capsule or lipstick is far below anything that caused trouble in safety tests.
Trouble often comes from context. Even drinking too much water leads to real health risks. What matters: Is something used within recommended limits, and do people have access to clear information about what’s inside? For those with allergies to related compounds, any product could be an issue. That’s not the chemical’s fault, but a reminder to pay attention to personal health history.
Transparency in labeling and regulatory oversight builds trust. I’m no stranger to reading research late at night after seeing some new chemical name on a cereal box. Authorities like the FDA, EPA, and European regulators study these ingredients with more resources than any one shopper. If scientists see signs of trouble, they issue alerts or change allowed concentrations. The track record plays out through reviews and real-world follow-ups. Each update shapes the conversation about ingredients.
For those with lingering questions, reaching out to a pharmacist or doctor helps take guesswork out of decision-making. They understand both individual health needs and the science behind additives. Open conversations bridge the gap between research lab and everyday kitchen table. With succinimide, decades of observation and testing point in one direction: Used in the right doses and situations, it doesn’t amount to a health risk for most people. Still, learning what’s in our products and medicines always pays off. Health works better when people feel informed, not just reassured.
Doctors sometimes prescribe succinimides, like ethosuximide or methsuximide, to treat absence seizures. These medications have been part of epilepsy care for decades. They don’t target all types of seizures, but for people dealing with short episodes of “blanking out,” succinimides can make a real difference.
Plenty of folks taking succinimides notice some changes they’d rather do without. Nausea pops up a lot. Kids and adults sometimes complain about an upset stomach, or even vomiting, after starting their pills. Some people get a strange sense of stomach pain or lose their appetite altogether. For kids already struggling to eat enough, that shift worries parents and doctors alike.
Drowsiness and feeling “off” mentally show up in patient stories too. Some days, it can feel like walking through mud—slower thinking, trouble focusing, even feeling dizzy when standing up. At times these moments pass, but for others, medication changes come into play.
On the tougher end, succinimides carry some rare but serious risks. Blood cell changes loom largest. Succinimides can drop the counts of white blood cells, red blood cells, or platelets. For instance, some people wind up with infections more easily or show up bruised after knocking an elbow on a doorframe. Doctors often order regular blood tests just to keep a lookout.
Skin rashes and allergic reactions can surprise people after they’ve already been on the medication for a while. One woman I spoke to had to switch to a different drug after developing a widespread itchy rash. Rash in a child with a fever always requires a quick doctor’s visit, because rare, severe rashes like Stevens-Johnson syndrome, though uncommon, can show up.
Changes in mood and behavior can also trouble families. Some people on these medicines report unexplained irritability, depression, or anxiety. I’ve met teenagers who couldn’t tell if their moods felt different because of school pressures or their pills. Parents and patients often need to advocate for themselves in these moments—making sure their care team knows about changes at home or school.
People can reduce the chance of problems with good medical follow-up. Most neurologists schedule routine blood tests and regular check-ins, not just at the start but through the course of treatment. Taking pills with food sometimes helps with nausea. If stomach problems linger, doctors may lower the dose or switch medications. Any big change in mood or skin needs a rapid response.
Open conversations create room for safer medication use. Some patients hesitate to talk about side effects for fear of judgement or losing access to medications that work. Honest back-and-forth builds trust, which makes it easier to find the safest, most effective plan.
Newer seizure medicines promise hope for those who can’t tolerate succinimides, but access to those drugs varies. Not everyone has insurance coverage for the pricier alternatives. Advocacy for wider access matters, both for kids and adults. Good epilepsy care means patients can talk openly about side effects and switch medications if needed, aiming for the best control with the least disruption.
Anyone who has worked with chemicals understands the value of a tidy, well-organized shelf. Succinimide doesn’t like chaos any more than you or I would. On a basic level, this white, crystalline powder usually sits in a chemical lab, waiting for its call in the next synthesis or pharmaceutical project. Some see storage as an afterthought, but a misstep here can end up spoiling months of careful planning—mixing work with trouble nobody needs.
Leaving succinimide out with the cleaning supplies or tucking it near a sunny window might seem harmless. The material can lose quality fast if it faces too much heat, humidity, or light. Elevated temperature speeds up decomposition and can result in yellowing and caking—problems not just for shelf appeal, but also for the chemical’s role in making accurate outcomes possible in reactions. As with many organic compounds, keeping it cool goes a long way in slowing unwanted chemical changes.
Experience shows that moisture is more sly than fire or sunlight. Even a sealed jar can betray you if the room’s air stays damp. Over time, succinimide absorbs water straight from the air, and this spells trouble for purity. Once mixed with too much moisture, you start to find it hard to weigh or prepare solutions with predictable results. You don’t have to take my word for it—a quick glance at lab records backs this up. Purity drops, and experiments go sideways.
Keep succinimide in an airtight, labeled container as your first line of defense. Choose bottles made from glass or high-quality plastic, and screw the lid down tight every single time. That habit beats any fancy container claims. A cool, dry cupboard, far from direct sunlight and heat sources, works better than most high-tech storage rooms unless the chemical calls for refrigeration, which regular lab-grade succinimide usually doesn’t require.
Dryness counts. Silica gel packets or other desiccants help keep things on the dry side. Toss a couple into the storage cabinet, not into the chemical jar itself. That trick picks up the slack if someone accidentally leaves the cap off or the room gets muggy. Check desiccant color dots every few weeks—when they change, swap them out. It’s a tiny routine with an outsized payoff in chemical stability.
Mistaken identity in a lab or workshop could tank research or trigger a safety mistake. Proper labeling takes care of more than just neatness. A clear label with the chemical name, concentration, date of receipt, and supplier means everyone in the lab stays on the same page. If a recall crops up, or a new team member joins, a label keeps confusion out of the game.
Lab safety slips into daily routine by taking a few careful steps with every bottle. Handling succinimide isn’t complicated if basic storage guidelines turn into habit rather than a rushed afterthought. Open containers one at a time, avoid sticking tools or scoops from other chemicals in the jar, and write down each time you use it. This kind of record can catch quality shifts before they snowball.
Poor storage choices ripple out—chemical quality drops, results get unreliable, and the risk of accidents climbs. Succinimide ought to last as long as the label says, with the same properties promised on day one. Sticking to proven storage steps turns out cleaner research, safer spaces, and less waste. Each bit of attention adds up in the long run, even if it involves just a twist of the lid or a scribble on a label.
Succinimide shows up in labs as a fairly simple but important compound. Take four carbon atoms, arrange them in a five-membered ring, drop in one nitrogen, and connect two double-bonded oxygens onto the ring. The result: a structure known as imide. More formally, chemists write its formula as C4H5NO2. The ring keeps it rigid, and those two oxygens help it pull off some interesting tricks in synthesis.
Seeing this ring for the first time in undergrad organic chemistry stuck in my mind. It looked innocent–like a little pentagon–yet held a spot in just about every pharmacology pathway chart I leafed through over the years.
Ask around in a pharmaceutical company’s research team, and succinimide’s name pops up quickly. Phenytoin or ethosuximide, both used in seizure treatment, come packed with derivatives of this small molecule. The imide ring structure offers stability that makes it suitable for drug design, especially if you look at how drugs need to survive long enough in the body to do their job.
In college, I saw a cousin cope with mild epilepsy. It’s hard to forget the relief in his parents’ voices after his doctor prescribed ethosuximide. Somewhere down the supply chain, chemists spent years tweaking that imide ring, optimizing it till it provided effective seizure control.
Beyond medicine, it finds its way into manufacturing as well. Industries use succinimide in corrosion inhibitors and as intermediates for dyes and agricultural chemicals. Add in its ability to react with nucleophiles, and it becomes a building block for peptide synthesis, which powers research in genetics and protein engineering.
I’ve noticed that its stable ring can sometimes lead folks to underestimate its hazards. Lab safety sheets flag succinimide as an irritant, so handling with care remains essential. Stories pop up every year about researchers suffering skin or respiratory irritation after treating succinimide too casually. Proper gloves, ventilation, and storage keep trouble at bay.
Sourcing also brings its own issues. Low-quality succinimide with impurities can throw off reactions, especially in drug design. I recall a project that delayed two months because an overseas shipment contained trace contaminants, causing unpredictable results in peptide coupling. Reliable sourcing–from labs following strict GMP (Good Manufacturing Practice) standards–cuts down headaches for people making medicines.
Strong regulation from FDA and better awareness among chemists help keep risks in check. Educators can play a bigger role in showing students not just the beauty of chemical structures but also real-world safety skills. Sharing near-miss stories and practical tips gives the next generation a chance to avoid repeating mistakes.
For sustainable chemistry, researchers have begun exploring ways to synthesize imides using greener solvents or recyclable catalysts. These efforts reduce environmental impact without sacrificing the utility that succinimide offers in advanced therapies or industrial applications.
In my work, I’ve learned that a molecule’s chemical skeleton isn’t just a diagram—it’s a story about medical breakthroughs, safer processes, and the generations of scientists building on each other’s work. Succinimide’s simple ring keeps giving us reasons to keep that story going.
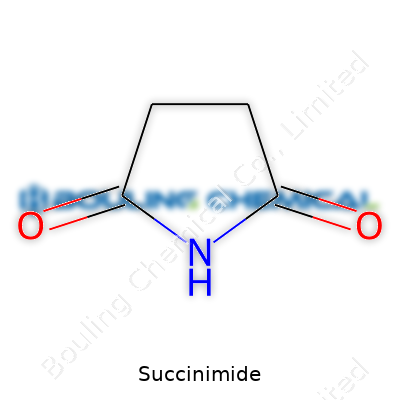
| Names | |
| Preferred IUPAC name | Pyrrolidine-2,5-dione |
| Other names |
Butanimide Amberlite Amberlite XAD-2 2,5-Pyrrolidinedione |
| Pronunciation | /ˌsʌkˈsɪn.ɪ.maɪd/ |
| Identifiers | |
| CAS Number | 123-56-8 |
| Beilstein Reference | Beilstein 60652 |
| ChEBI | CHEBI:45549 |
| ChEMBL | CHEMBL1406 |
| ChemSpider | 5486 |
| DrugBank | DB00751 |
| ECHA InfoCard | 100.007.760 |
| EC Number | 205-025-8 |
| Gmelin Reference | 6071 |
| KEGG | C00732 |
| MeSH | D013429 |
| PubChem CID | 8063 |
| RTECS number | WN3675000 |
| UNII | 7M87SW2I3E |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C4H5NO2 |
| Molar mass | 99.09 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.19 g/mL |
| Solubility in water | Moderately soluble |
| log P | -1.18 |
| Vapor pressure | 0.0055 mmHg (25°C) |
| Acidity (pKa) | 9.5 |
| Basicity (pKb) | 11.38 |
| Magnetic susceptibility (χ) | -52.0e-6 cm³/mol |
| Refractive index (nD) | 1.485 |
| Viscosity | 13.7 cP (20°C) |
| Dipole moment | 3.73 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 160.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -400.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1797.8 kJ/mol |
| Pharmacology | |
| ATC code | N03AB04 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Danger |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements: "P261, P280, P304+P340, P312, P405, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 154°C |
| Autoignition temperature | 800°C |
| Explosive limits | Not found. |
| Lethal dose or concentration | LD50 oral rat 5000 mg/kg |
| LD50 (median dose) | 1680 mg/kg (Rat, oral) |
| NIOSH | SN122 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 15-40 |
| Related compounds | |
| Related compounds |
Maleimide Phthalimide Glutarimide Barbituric acid Hydantoin |