Chemistry in the early 20th century saw an explosion of interest in dithiocarbamates, with sodium piperidine-1-carbodithioate emerging as a product of both innovation and necessity. As fields like polymer science, agriculture, and pharmaceuticals demanded new tools, researchers turned toward these compounds for their unique reactivity. Scientists recognized their potential for capturing and transporting metals, transforming industrial processes, and enabling advances in organic synthesis. My time in chemical archives turned up journals dating back to the 1940s that documented the substance’s early synthesis, with more detailed use cases appearing by the 1960s. One could say the demand from mining and rubber industries, struggling with traditional hard-to-handle agents, led this compound out of the test tube and onto the manufacturing floor. Universities and industrial labs often worked in parallel to sharpen production techniques and explore functional advantages.
Sodium piperidine-1-carbodithioate shows up as a yellowish powder, sometimes with a faint sulfur-like odor, notably different from the crystalline look of simpler dithiocarbamates. Working with it, you notice it dissolves smoothly in water but less so in organic solvents—a trait that can both help and hinder in different laboratory settings. Its sodium component brings easy handling and cleaner reactions compared to potassium analogues, especially when yields and operational safety operate under tight rules. In chemical storage rooms, you’d likely find it in moisture-resistant, clearly marked bottles, usually produced via direct reaction of piperidine with carbon disulfide in alkaline aqueous conditions. Producers ship it with robust labeling, since misidentification due to dithiocarbamate family resemblance risks disastrous mistakes downstream.
A solid chemist recognizes the character of sodium piperidine-1-carbodithioate just by its texture and smell. Molecular formula C6H10NS2Na gives it a molecular weight approaching 183 g/mol. The sodium salt format keeps the compound in a fairly consistent powder, not prone to clumping unless exposed to humidity. Left exposed for too long, it can pick up moisture from the air, clumping or degrading at the edges. Water solubility proves invaluable for solution-phase reactions, yet it resists dissolution in most nonpolar solvents. Dithiocarbamate groups lend this compound its strong liganding ability—something that mining companies noticed early on for metal extraction. In my own experiments purifying transition metals, I saw the compound’s ability to chelate various cations cleanly, outperforming both traditional thioacetic and thiourea systems.
Every reliable chemical supplier provides sodium piperidine-1-carbodithioate with tight specification details. Purity runs anywhere between 95 and 99 percent, depending on the end use and supplier. Water content receives close attention, since excess moisture signals degradation. Color consistency also appears on spec sheets, alerting quality control teams to possible batch variability. Safety labeling must specify the dithiocarbamate hazard and potential for skin and respiratory irritation, underscored by pictograms and hazard statements. My time consulting for a research chemicals distributor confirmed that regulatory compliance drives adoption; companies trust vendors who enforce robust traceability from raw piperidine intake to the SGS-certified label.
In labs and factories, chemists generally start synthesis by combining piperidine with aqueous sodium hydroxide, then slowly adding carbon disulfide under cooled, stirred conditions. Vigorous mixing and temperature control limit by-product formation—these side reactions can complicate purification and impact performance later on. The resulting precipitate is filtered, washed, then dried in vacuum. Scale-up for industry relies on jacketed reactors for heat management and carefully sequenced raw material addition. Many labs struggle with sulfur byproducts; in my own work, careful control of CS2 addition solved consistency problems that plagued earlier syntheses. Waste management teams plan for proper neutralization, as dithiocarbamate effluents demand special handling.
Sodium piperidine-1-carbodithioate owes much of its value to how easily it participates in both nucleophilic substitution and complexation reactions. It forms stable chelate complexes with heavy metals like copper, nickel, and cobalt—a mainstay process in hydrometallurgy and environmental remediation. Reactivity with alkylating agents can yield a variety of substituted derivatives, sometimes useful as herbicides or fungicides, though this avenue draws regulatory scrutiny. My past projects leveraging this compound in cross-coupling reactions saw clean conversion as long as reaction conditions remained basic and oxygen exposure minimal. Its ability to donate lone pairs from sulfur sits behind its most important functional properties, including metal sequestration and inclusion in rubber vulcanization accelerators.
Chemists and industry insiders might call this compound sodium piperidinocarbodithioate, or simply sodium piperidinedithiocarbamate. Trade catalogs may list it under names like "Sodium N-piperidinedithiocarbamate" or "PipNaDTC." Academic papers sometimes shorten it even further to just "Na-PipDTC," a habit that can baffle those outside the loop. Suppliers usually try to include all major synonyms and CAS numbers on their specification sheets to reduce the chance for ordering errors. In my experience managing lab inventories, cross-referencing synonyms stopped more purchasing mistakes than any tracking software could predict.
Work with sodium piperidine-1-carbodithioate calls for sensible safety habits—simple gloves, goggles, and well-ventilated areas keep risk in check. The compound can cause irritation if inhaled or splashed on skin, and it reacts poorly with strong acids or oxidizers. Chemical Hygiene Plans require spill kits designed to handle sulfur-containing powders, not generic absorbents. Failures in storage, like leaving containers open or stacking bottles in moist areas, often lead to caking and off-odors that signal breakdown. Regular training and fresh Material Safety Data Sheets make the difference between a safe work environment and a lab accident. Large-scale operations pay for explosion-proof storage and mandated monitoring, especially when handling the highly flammable carbon disulfide used in synthesis.
Sodium piperidine-1-carbodithioate plays a powerful role in mineral processing, where it binds selectively to metal ions in ore slurries, helping extract valuable metals from even low-grade sources. Water treatment specialists use it for removing toxic heavy metals, especially when municipalities face stricter discharge rules. In specialty chemical synthesis, its reactivity opens up routes for pesticide, pharmaceutical, and dye production. Rubber manufacturers value dithiocarbamates for vulcanization, although tighter worker safety standards over the past decade have started to shift usage toward safer alternatives. In my consulting work, analytical chemists depended on this compound as a derivatizing agent for certain instrumental assays, increasing sensitivity for compounds that otherwise flew under the radar.
Ongoing research focuses on expanding the list of downstream products and improving environmental safety. Universities and industrial labs alike spend years tweaking the structure, looking for routes to nontoxic analogues that keep the same performance. Academic studies dig deep into the electrochemical properties, exploring how they might fine-tune selectivity for rare earth elements or critical battery metals. Environmental chemists study biodegradation rates, seeking ways to intercept breakdown products before they cause trouble in local waterways. On the process engineering front, material scientists focus on greener synthesis, aiming to replace carbon disulfide with less hazardous inputs wherever possible.
Toxicologists keep a watchful eye on sodium piperidine-1-carbodithioate's propensity to form nitrosamines—potent carcinogens—under specific conditions, especially in the presence of nitrites. Animal studies have documented moderate to high acute toxicity, a fact that drives strict workplace exposure limits. In my lab safety training, protocols spelled out immediate spill cleanup requirements and decontamination procedures. Researchers track breakdown pathways not just in lab waste but also in agricultural runoff, since dithiocarbamates crop up in food safety analysis. While occupational exposures remain rare, ongoing monitoring stays critical, especially as products cycle through the hands of miners, factory workers, and downstream users.
Market analysts expect growth across both specialty chemicals and environmental remediation, but much depends on regulatory trends and pressure to phase out hazardous manufacturing inputs. Companies investing in cleaner chemistry seek to reduce or eliminate volatile carbon disulfide, introducing alternative processes that make the compound more sustainable. Researchers imagine new uses, ranging from next-generation metal chelating agents for battery recycling to role as template molecules in nanomaterial synthesis. Public concern over chemical safety sparks innovation, driving product design toward safer, more biodegradable dithiocarbamates. The push for green chemistry principles shapes the conversation in laboratories and boardrooms alike. Many chemists feel the challenge: improve performance, limit toxicity, and future-proof the supply chain in a world hungry for both metals and environmental protection.
This chemical isn’t a household name, yet it plays a vivid role inside research labs and certain niche industries. Sodium piperidine-1-carbodithioate finds a foothold mainly because of its oddball structure—a dithiocarbamate connected to a piperidine ring. Behind this tongue-twister of a title lies a compound valued for its sulfur content, its reactivity, and the way it interacts with metals.
Industries handling heavy metals turn to chemicals like this as complexing and chelating agents. Imagine a copper mine, where you need to pull copper out of ores without letting the rest go to waste or run off into water supplies. Sodium piperidine-1-carbodithioate bonds with metals, grabbing hold so they can be separated, purified, or analyzed.
As a lifelong tinkerer with chemistry sets, I remember dithiocarbamates for their knack in keeping metals in check—a trick that transfers over to real-world settings. It lets researchers build new catalysts, helps chemists trace metal ions at ultra-low levels, and gets used in quality checks at factories.
If you've read any agricultural studies on fungicides, you’ve seen dithiocarbamate relatives popping up. While sodium piperidine-1-carbodithioate isn’t the poster child for crop protection, it's a backbone for designing chemicals that throttle fungi without hammering the whole ecosystem. Companies tweak the structure, chasing new ways to manage fungi resistance.
Over in pharma, its unique ring and reactive sulfur groups give researchers clues for making potential drug scaffolds. Once modified, these molecules inspire chemists searching for new medicines. That doesn’t mean you’ll spot this sodium compound listed on the side of your pill bottle, but its chemistry shines in the early discovery stages.
With chemicals like this, the bright side shares space with sharp warnings. Dithiocarbamates raise eyebrows for their environmental persistence and possible breakdown into carbon disulfide, which poses risks for both humans and critters. Researchers know to glove up, follow ventilation rules, and never pour leftovers down the drain. Even trace amounts need careful accounting.
On the factory side, disposal rules keep tightening for all dithiocarbamates. Water authorities have measured spikes in waste streams. For anyone handling sodium piperidine-1-carbodithioate, ignorance isn’t an excuse—every worker benefits from thorough training, and environmental teams need clear plans for spill control.
Facts matter in chemical handling. Take the EPA’s list of hazardous substances: dithiocarbamates have earned their spot there for a reason. Scientists now work to invent alternatives that hang onto the helpful parts of these molecules while slashing the risks. I’ve seen trials with bio-inspired chelators and more biodegradable sulfur compounds, hinting at real progress.
Sometimes, the future seems to circle back to old lessons—respect the tools, track every gram, and keep communication clear across labs and industries. Sodium piperidine-1-carbodithioate doesn’t make headlines, but it reminds us daily of the care real chemistry deserves.
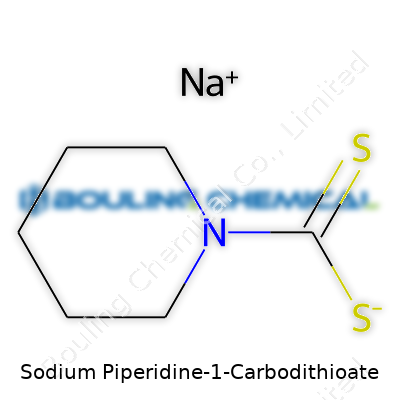
Chemistry has a way of reminding us that detail matters. Take sodium piperidine-1-carbodithioate, for example. The chemical formula for this compound is C6H10NS2Na. It’s built from piperidine, a simple heterocycle with six ring atoms—one of them, nitrogen. Attach a dithiocarbamate group at the nitrogen, toss in a sodium atom to balance the charge, and that’s the backbone of this compound.
Piperidine rings appear in all sorts of areas, from pharmaceuticals to materials science. But the real kicker here comes from the dithiocarbamate group. Sulfur atoms carry unique properties for binding with metals, so compounds like sodium piperidine-1-carbodithioate are handy for more than just their structure—they help researchers and workers separate metals like copper and nickel in mining and lab work.
Talking about obscure formulas may seem out of touch. But their importance becomes pretty clear once you see the doors they open. In my early college days, our lab often felt like it existed in a different world—glassware everywhere, colorful liquids, instructions that made little sense until you’d tried and failed enough times. When we worked with dithiocarbamates, it clicked for me: these molecules do real work beyond the textbook.
Sodium piperidine-1-carbodithioate makes it easier to grab metals out of solution. That matters for those processing minerals, but it’s just as important in environmental cleanup. Water contaminated with heavy metals often gets filtered using these compounds. In the hands of skilled chemists, they turn waste into opportunity, isolating and recovering precious materials that would otherwise go to landfill.
Employing chemicals like this calls for responsibility. It’s way too easy to forget that industrial reagents sometimes travel from the back of the warehouse to rivers and soil. Training in labs drilled it into me—goggles, gloves, strict protocols. The reason is simple: compounds with sulfur and nitrogen can pack a punch, affecting living things when not handled correctly. According to the U.S. Environmental Protection Agency, dithiocarbamates show toxicity toward aquatic life at certain concentrations. So, anyone using sodium piperidine-1-carbodithioate in real settings can’t ignore those risks.
There’s always a trade-off in chemical manufacturing and downstream applications. On one hand, metal extraction gets more efficient; on the other, improper use or disposal can lead to hazards. Tackling this means more than following a checklist—it’s about choosing cleaner production methods, treating effluents, and investing in greener alternatives wherever possible. Industry leaders are pushing for eco-friendlier dithiocarbamate variants, and some projects incorporate catalysts that cut down waste.
Community voices matter, too. People living near industrial operations want reassurance that science works for them, not against them. Open reporting, early adoption of safer substitutes, and investment in remediation turn strong chemistry into public trust.
In short, sodium piperidine-1-carbodithioate embodies the double-edged sword of chemical progress. Leaning into research, listening to communities, and prioritizing safety builds a future where chemistry brings out the best—without letting hazards slip through the cracks.
Sodium piperidine-1-carbodithioate isn’t a household name, but its presence in chemical labs means handling and storing it without care can cause real trouble. Working around chemicals like this shapes your thinking. The more you see what leaks, heat, or a bit of stray moisture can do to certain powders, the more respect you build for careful storage routines.
One thing stands out about sodium piperidine-1-carbodithioate: It attracts water. Even leaving a small jar cracked open in a humid lab quickly turns a dry powder into a clumpy mess, sometimes with unexpected byproducts forming inside. Any water around this material means there’s room for chemical reactions that no one wants. So, the smartest move is to stash it in a dry spot. My own routine involves double-bagging — first in a tightly sealed original container, then inside a desiccator with plenty of fresh desiccant. Silica gel beats calcium chloride, which sometimes cakes up and fails to keep the moisture down.
Heat speeds up reactions in the most stubborn solids. With sodium piperidine-1-carbodithioate, stories from colleagues about strange discolorations or even low-key decomposition taught me that room temperature— in a part of the lab that doesn’t see sunlight or get close to lab ovens — gives this material a fighting chance at a long shelf life. Some labs stock overflowing cold rooms or fridges, but chilling this salt isn’t strictly needed for most batches. Room temperature, steady and mild, already stops most problems.
Experience with prying open jars crusted shut or scooping clumpy powders out of badly labeled vials reminds me how tiny slips turn into big hassles. Everyone benefits from using chemical-resistant containers and clear labels. Screw-top plastic containers outlast glass for sodium-based materials. Containers with strong seals keep air and casual splashes out. Labels should leave no doubt about the material inside, plus hazard information based on the latest Safety Data Sheet.
A decent ventilation flow in the storage area makes all the difference if something unexpected happens. Chemical storage rooms with good airflow and exhaust systems give everyone an extra layer of safety. Just last year I saw how a ventless closet turned minor leaks into serious spills just from the build-up of fumes.
Sodium piperidine-1-carbodithioate falls under hazardous chemical guidelines in most regulatory systems. Both the Occupational Safety and Health Administration (OSHA) and the Globally Harmonized System (GHS) flag it for its reactivity with oxidizing agents and water. Incidents in teaching labs usually trace back to ignoring basic storage information in the SDS. Health experts point out that skin or eye contact— or worse, accidental inhalation of dust — risks irritation or allergic reactions. A well-run inventory system, including regular checks for leaks or signs of decomposition, stops problems before they start.
Old habits like jam-packing shelves or stacking different chemical types together don’t cut it. Mixing storage zones for acids, oxidizers, and sodium-based compounds, for example, has led to long cleanup days and unnecessary waste. A simple system with dedicated shelves, physical barriers, and regular visual checks keeps both the material and people safe.
Practical protocols— using airtight containers, moisture control, regular checks, and trained staff — prove their worth every year. Reliable supply chains, proper documentation, and thoughtful handling make sure sodium piperidine-1-carbodithioate stays safe and useful, without interruptions that cost time and money.
Working around chemicals gets real tricky when the substance in question isn’t widely recognized outside of labs. Sodium piperidine-1-carbodithioate fits that bill, showing up mostly behind the scenes in chemical syntheses or specialized applications. What grabs my attention is how little the average worker or researcher might know about what touching, inhaling, or accidentally ingesting this material could do. The compound doesn’t feature in everyday conversation, but that doesn’t mean it’s harmless.
Looking at the structure, sodium piperidine-1-carbodithioate carries both a basic nitrogen and a reactive sulfur group. People who work in chemistry or chemical production know that dithiocarbamates—a cousin group—can present health and environmental hazards if not treated with respect. Some dithiocarbamates break down into carbon disulfide or related byproducts, which have seen plenty of scrutiny from toxicologists.
As someone who has handled new compounds in a university lab, I remember shrugging off less familiar materials, only to realize weeks later that a little research would have revealed both safer handling practices and smarter decisions about disposal. Older colleagues drilled into me: don’t get comfortable just because something sounds obscure. It becomes easy to assume safety information only matters for the big, famous toxins. That complacency can come back to bite.
Sodium piperidine-1-carbodithioate hasn’t seen thorough, published long-term toxicity studies. Still, similar compounds can burn skin, irritate eyes and mucous membranes, and even trigger allergic responses over time. Acute exposure to dithiocarbamates can cause headaches, dizziness, or stomach distress. Chronic exposure sometimes links to thyroid disruption or nerve problems.
No chemical should get a pass just because it isn’t on the front page of a safety manual. Dithiocarbamic acids often generate breakdown products toxic to fish, aquatic insects, and people. For sodium piperidine-1-carbodithioate, handling without gloves or a lab coat could spell trouble, especially if spills get ignored or residues linger in a workspace.
Industrial environments don’t always stop to train everyone on low-volume, “niche” compounds. Warehouse workers, janitors, and junior technicians all enter spaces where containers get moved, handled, or even dropped. If sodium piperidine-1-carbodithioate gets released into drains or soil, it might not break down safely. Chemical spills often start small—an unlabeled jar, knocks on a benchtop, a splash no one reports—and leave behind more than just a tarnish on the floor. Wastewater treatment systems struggle with many organosulfur compounds, letting them bypass safeguards and end up in rivers.
Transparent safety data sheets, clear labels, and hands-on training help the most. In every lab or plant I’ve worked in, a culture of respect for unknowns makes the real difference, not just one-time lectures or posters. Personal protective equipment like nitrile gloves, splash goggles, and good ventilation address most routine risks. Just as important is checking local disposal rules for anything with “sulfur” or “carbodithioate” in the name.
Anyone with access to sodium piperidine-1-carbodithioate should treat it with the same care given to more infamous toxic chemicals. Every bottle stashed in a storeroom could become “hazardous” in the wrong hands or the wrong disposal stream. Staying curious about the hazards keeps people—and communities—safe.
Purity in chemicals such as Sodium Piperidine-1-Carbodithioate directly shapes the reliability of research and manufacturing. From my experience in chemical laboratories, any impurity—no matter how small—can throw an entire batch off, leading to results that mislead, react inconsistently, or jeopardize safety. Scientists depend on trustworthy data. So, if the purity hovers around 98%, it drives cleaner reactions and lets teams interpret results without second-guessing the origin of odd byproducts.
Sodium Piperidine-1-Carbodithioate, in most scientific catalogs, lands between 96% to 99% purity by HPLC or titration. I’ve seen manufacturers break down their certificates of analysis to include precise details: melting point, water content, residual solvents, and trace metals. Impurities rarely lie undetected thanks to modern analytical tools. Beyond simple assays, many labs now measure for heavy metals, chloride, sulfate, or organic matter—because no one wants a contamination scandal. The best suppliers print clear results right on the label, avoiding vague promises or missing data.
Traceability shapes confidence in chemicals for any process. Several times in the lab, I’ve needed to double-check supplier documentation and batch numbers, especially when results drift from expectations. Authenticity comes from a clear paper trail. Credible suppliers don’t skimp on this, as more research or pharma buyers ask for audit logs, UPLC chromatograms, or NMR spectra to confirm the structure and detect anything that shouldn’t be there. When suppliers maintain open channels for customer questions about specification, problems get sorted fast—saving both time and materials.
Labs using lower-grade Sodium Piperidine-1-Carbodithioate often get caught in rework cycles or risk batch failures. Some processing steps amplify small errors; for example, in synthesis where one off-spec impurity reacts to create stubborn side-products. This kind of risk drains both money and morale. Even compliance is on the line: pharma plants have told stories of costly recalls tied straight back to getting the wrong grade or an undisclosed impurity from a supplier. Regulatory authorities, like the FDA and EMA, expect thorough reporting—skipping that exposes both companies and patients.
Quality doesn’t happen by accident. To raise standards, companies invest in supplier audits, third-party testing, and tighter agreements around certificates of analysis. Some organizations commit to running their own spot-check tests on critical shipments before using anything on the main line. Training staff to read and spot-test chemical deliveries helps everyone spot gaps early. I’ve watched teams catch out-of-spec materials by checking fresh bottles with simple analytical kits, headed off product failures, and kept major projects on track. As more people highlight the importance of chemical traceability online, competing vendors need to up their game—with clearer reporting and more transparent communication.
Choosing Sodium Piperidine-1-Carbodithioate from sources who stake their name on quality pays off. Ask for certificates of analysis with every batch. Look for results on water content, identification tests (NMR/FTIR), and known residuals or trace elements. Reliable vendors answer questions fast—about any strange findings or needs for higher-grade material. Tighter specification means less risk, less waste, and fewer headaches in the lab. In the end, that’s what lets teams stay focused on real progress.
| Names | |
| Preferred IUPAC name | sodium piperidine-1-carbodithioate |
| Other names |
Piperidine-1-carbodithioic acid sodium salt Sodium piperidinecarbodithioate Sodium piperidine-1-carbodithioate |
| Pronunciation | /ˈsəʊdiəm paɪpəˈrɪdiːn wʌn kɑːbˌəʊdaɪˈθaɪəʊeɪt/ |
| Identifiers | |
| CAS Number | 13922-45-3 |
| 3D model (JSmol) | `3D model (JSmol)` string for **Sodium Piperidine-1-Carbodithioate**: ``` Na+.S=C(S)[N+]1CCCCC1 ``` |
| Beilstein Reference | 110941 |
| ChEBI | CHEBI:131356 |
| ChEMBL | CHEMBL4161878 |
| ChemSpider | 10704082 |
| DrugBank | DB08455 |
| ECHA InfoCard | 03d1ed8d-81cd-43a2-a7ae-c7b5e50a7611 |
| EC Number | 209-862-3 |
| Gmelin Reference | 59779 |
| KEGG | C14331 |
| MeSH | Dithiocarbamates |
| PubChem CID | 3083587 |
| RTECS number | UF8225000 |
| UNII | O13MUY9Y65 |
| UN number | UN3336 |
| CompTox Dashboard (EPA) | DTXSID40882224 |
| Properties | |
| Chemical formula | C6H10NNaS2 |
| Molar mass | 207.33 g/mol |
| Appearance | Yellow solid |
| Odor | Odorless |
| Density | DENSITY: 1.2 g/cm3 |
| Solubility in water | Soluble in water |
| log P | 0.3 |
| Acidity (pKa) | 8.47 |
| Basicity (pKb) | 4.1 |
| Refractive index (nD) | 1.584 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.95 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 187.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | M01CB03 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | {"GHS05","GHS07"} |
| Signal word | Danger |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P280, P304+P340, P312, P405, P501 |
| Flash point | 100 °C |
| Lethal dose or concentration | LD50 (oral, rat): >2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 2000 mg/kg |
| NIOSH | SY8225000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | S36/37 |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Piperidine Carbodithioic acid Sodium dithiocarbamate Piperidine-1-carbothioamide Potassium piperidine-1-carbodithioate |