Sodium Benzothiazol-2-Yl Sulphide entered chemical industry circles decades ago, and it quickly caught attention as a backbone ingredient in the rubber sector, especially after the surge of vulcanization in the early 20th century. Chemists started to notice its potential for speeding up vulcanization reactions, transforming raw rubber into durable materials. Accounts from technical literature in the 1930s mention how this compound made a measurable difference in both performance and production speed. Early research houses, particularly in Europe and North America, moved swiftly to patent its use, leading to broad adoption through the automotive and industrial growth years. Lab notebooks and old patents serve up a straightforward lesson: this compound’s history is built on finding more efficient ways to meet real-world challenges in manufacturing tires and industrial hoses, impacting economies and daily commutes.
People in chemistry labs toss around several names for this chemical, including Sodium 2-Benzothiazolyl Sulfide, Benzothiazole-2-thiol sodium salt, and sometimes just “NaBT.” In simplest terms, this is a sodium salt derivative from benzothiazole chemistry, with sulfur thrown into the mix to give it the punch manufacturers rely on. Manufacturers rely on its yellow to brown crystalline or powdery form, which they ship in drums or bags lined with anti-static coatings to keep it safe on the move. Product labels list out the content, moisture limits, and purity levels, usually hovering at or above 99%. Quality handlers stamp labels with recommended storage conditions and clear batch numbers for full traceability.
This chemical tends to show up as a pale yellow to light brown solid. Its solubility in water comes in handy for blending into aqueous solutions during manufacturing. The smell gives away its sulfur roots, sometimes described as faintly pungent. Chemically, it stands steady in alkali conditions, but doesn’t fare well around strong acids or oxidizers, which can break down the benzothiazole ring or release noxious gases. The sodium content helps it dissolve and disperse where needed, while the benzothiazole core resists breakdown at higher processing temperatures common in rubber mixing. The melting point hovers in the range of 90-120°C, and it starts to decompose before any significant melting occurs. Its pH in water stays safely on the alkaline side, usually above 10, helping technicians adjust mixtures without tugging the overall pH out of a workable range.
Every batch gets analyzed using established methods like HPLC or UV-Vis absorption techniques. Specifications outline minimum purity (not less than 98 or 99%), moisture content (generally under 1%), sodium percentage, and residual organics. Regulatory labeling, as required under GHS and REACH, highlights health and environmental hazards, precautionary pictograms, signal words such as “Warning,” and tightly phrased instructions for transport, storage, and emergency procedures. Labels give clear expiry dates, batch codes, and sometimes QR codes for digital tracking. I’ve seen facility audits where missing any of these details led to a shutdown until compliance was restored, underscoring the real-world value of seemingly dry documentation. Tech data sheets also give mixing and compatibility advice, based on decades of accident investigations and bench chemistry.
The go-to route for making Sodium Benzothiazol-2-Yl Sulphide starts with benzothiazole. Manufacturers run the reaction with elemental sulfur and sodium-based bases at moderate temperatures, usually around 120-130°C. The setup needs reliable agitators, sturdy glass-lined reactors or stainless steel vessels, and venting for any hydrogen sulfide produced along the way. After the crude reaction has settled, technicians filter the product, wash it to remove leftover reagents, and dry it at controlled temperatures. Good labs use analytical checks at every step—both for safety and to boost yields. Many companies have switched to closed-loop systems with real-time sensors, not only to protect workers from fumes, but also to squeeze every last bit of useful product from each batch. Process tweaks have cut down on waste and energy use, important in regions with strict environmental oversight.
In the lab, Sodium Benzothiazol-2-Yl Sulphide often gets tapped for its free sulfur, which comes alive under heat or pressure. This sulfur activity lets it connect—often quite literally—with unsaturated bonds in raw rubber, building bridges that dramatically change the rubber’s mechanical and thermal properties. The benzothiazole ring offers sites for further tweaking, such as halogenation, methylation, or oxidation. Some researchers experiment with salty substitutions, swapping sodium for potassium or lithium to spin out tailored catalysts for different industries. At high concentrations, this chemical can bump up reaction rates, but at a cost: more rapid exotherms, risk of scorch, or changes in final product properties. Figuring out the sweet spot for use remains part art, part science, handed down by shop-floor veterans and young chemical engineers both learning on the job.
You may see it listed as Sodyum 2-Benzotiazolil Sülfit, Benzothiazolethiosodium, or by trade names pitched by multinational suppliers or regional sellers. Sometimes catalogues shorten it to NaBT or BT-Na. In markets like India or Turkey, local suppliers run with translations or company-specific monikers, while global chemical indexes stick to IUPAC convention. In my experience, the same substance often circulates under different names, leading to some confusion or even double-ordering unless procurement teams dig into the actual structure or specifications. Seasoned professionals always double-check by CAS number to avoid costly mix-ups.
Anyone who’s handled Sodium Benzothiazol-2-Yl Sulphide learns the hard way: a lapse in PPE or sloppy storage can mean skin rashes, breathing trouble, or worse. Standard operating procedures call for full nitrile gloves, chemical goggles, and local exhaust in even small labs. Whole plants run under strict ventilation codes. Safety Data Sheets flag chronic exposure risks and urge prompt decontamination after spills. Regulations think long-term too, with rules on waste disposal, spill response kits, and air emissions. Facilities train everyone, from forklift drivers to warehouse managers, in emergency protocols. This isn’t just bureaucracy—OSHA inspections and insurance reviews often trace incidents back to skipped safety steps or poor labeling. Even routine cleaning now includes periodic air sampling, and most sites ban open flames to dodge risk from volatile sulfur gases. Keeping up with new exposure research, facilities sometimes pilot air scrubbers or updated masks after press reports about new health findings.
Nearly every tire made today has a bit of chemistry from Sodium Benzothiazol-2-Yl Sulphide baked in. The compound’s ability to speed up sulfur cross-linking in rubber has cut production times, improved durability, and boosted resistance to weathering—in turn leading to safer, economical tires and conveyor belts. Operators also put it to work in cable insulation, gaskets, industrial hoses, and even latex products. Some specialty fields, like advanced adhesives or oil-well swelling agents, experiment with it for unique performance tweaks. Facilities often stagger production schedules just to keep enough on hand for critical processes, especially in big automotive supply chains.
Research groups both in academic and industry settings look into new tweaks to the sodium and thiazole core, hoping to squeeze out better performance or less environmental impact. Some labs experiment with greener solvents or milder reaction conditions to reduce byproducts and hazardous waste. Computational modeling now helps predict how substitutions can shift reactivity, allowing teams to run trials on the computer before scaling up actual synthesis. Regulatory pressure from Europe and North America has driven a wave of R&D funding focused on “green rubber accelerators.” Young scientists often bring fresh eyes to legacy processes and spot surprising ways to cut raw material costs, recycle off-spec batches, or recover valuable byproducts. Cooperative work between universities and chemical producers has already paid off in cleaner prep routes and more stable formulations that keep for longer in storage.
Journal articles since the 1970s raise questions about both acute and chronic risks. Animal studies suggest repeated exposure can cause skin sensitization, irritation of mucus membranes, or impact the liver or kidneys at high doses. Newer work zeros in on breakdown products, such as benzothiazole derivatives and sulfur oxides, which can turn up in the environment around rubber plants. Environmental scientists watch closely for water contamination, and some localities have restricted use or increased wastewater monitoring as benzothiazole structures have appeared in rivers and fish tissue. Responding to this, regulatory agencies urge lower workplace exposure limits and more comprehensive monitoring. Manufacturers respond with investment in hazardous material training and regular health screenings. Research continues, with new data trickling out every year in journals focused on industrial hygiene and environmental toxicology.
Chemical plants and research labs know demand won’t drop off soon, with global mobility and infrastructure projects chewing through millions of tons of rubber products. At the same time, environmental rules grow stricter yearly, forcing producers to hunt for preparation routes with less waste and lower solvent footprints. There’s push and pull between performance demands and regulatory barriers, driving companies to look deeper into biodegradable alternatives or safer analogs. Automation, predictive modeling, and better process controls may soon squeeze more output with less risk and environmental impact. Conversations at industry conferences turn to circular economy models, with some startups betting on techniques to recover or recycle key benzothiazole units from end-of-life tires. Eventually, public concern over persistent chemical residues may tip the market toward safer, more sustainable versions or trigger fresh innovation in this old corner of chemistry.
Sodium benzothiazol-2-yl sulphide shows up most often in places you don’t look twice at—tire plants, rubber factories, sometimes in warehouses stacked with raw chemicals. If you’ve ever handled a tire and caught its sharp, almost chemical smell, you’ve come into contact with a product that relies on this compound. It’s used as an accelerator in the vulcanization of rubber. Vulcanization is a big deal in making tires or anything that’s supposed to go through rough use and hold up without cracking or falling apart.
Rubber alone does not last. It wears out, loses elasticity, and can become brittle in no time. Factories use sodium benzothiazol-2-yl sulphide to help sulphur bond to the rubber more quickly and more completely. You get tires that grip the road better and boots that hold out against rain and mud. Stretches of conveyor belts in supermarkets, even seals in washing machines, turn to this chemical for durability. Without these qualities, products break down faster, leading to more waste, higher costs, and more frequent replacements.
Handling chemicals in industry calls for hands-on experience. I’ve spent days touring plants where workers gear up with gloves, goggles, and proper ventilation. Sodium benzothiazol-2-yl sulphide can be harmful if someone ignores safety. Skin contact or inhalation might cause irritation. Proper training, storage, and monitoring ensure it doesn’t turn from a helpful ingredient to a workplace hazard.
The biggest concern pops up around disposal and environmental impact. Runoff from factories, if not managed, can slip into local streams and soil. Reports from the U.S. Environmental Protection Agency show that benzothiazole derivatives can linger in the environment and affect aquatic life. Factories must treat their waste before it hits the drains and should regularly audit their containment systems.
The world keeps searching for methods that keep workers safe and ecosystems healthy. I’ve read about researchers in Germany and Japan who test alternative chemicals that give rubber its strength without posing as much risk. Some companies have moved to closed processing systems that limit exposure and leaks. It’s not an easy switch since new methods need to work just as well, or better, than existing ones to convince manufacturers to change.
Communities close to manufacturing sites ask questions—what’s in the water, how do nearby schools stay safe, who’s responsible if something goes wrong? Regulators and businesses must answer these concerns with clear reports and real monitoring. My experience says transparency helps, especially when people know that oversight doesn’t get brushed aside.
Products using sodium benzothiazol-2-yl sulphide last longer. You notice it not just in cars but in everyday items—like the rubber grip on your bicycle handlebar or the mat under your dog’s bowl. Factories depend on chemicals like this to meet demands for toughness and reliability. The key is balancing industrial benefit with human health and the planet’s future. That’s something worth tracking, whether you’re in the field or just looking for boots that don’t spring leaks after a single season.
Sodium Benzothiazol-2-Yl Sulphide pops up often in rubber manufacturing and some specialized processes in water treatment. It reacts strongly with moisture and acids, releasing toxic fumes. This trait makes it tricky if the basic safety steps slip through the cracks.
No matter how busy the day gets, bypassing gloves or lab coats invites trouble. Simple, tight-fitting nitrile or rubber gloves shield hands from even minor spills. Goggles with side shields keep eyes protected from vapors and accidental splashes—there’s no making do with regular eyeglasses. Laboratory coats, closed-toe shoes, and long pants give a basic level of protection, and wearing a mask, preferably a chemical respirator, keeps you from breathing in dust or fumes. Skin, eyes, and lungs pay the first price when things go wrong.
An experience worth sharing: overhead exhaust hoods cut odor and exposure to vapors dramatically, especially with finicky chemicals like this one. Trying to handle small amounts outside proper ventilation feels like inviting a headache or worse. Ventilation runs as the unsung hero—simple open windows or desktop fans just can’t compare to a certified fume hood. Always prep and weigh this chemical inside one.
Chemicals find their way into the wrong spot far too often. Labels need to stay clean and easy to read so no one mistakes a hazardous substance for something benign after a rushed shift. Secure containers, tightly closed, kept dry and away from any source of water, tell their own story: don’t trust a shelf above the sink, don’t mix it with acids or oxidizers. Storing in a cabinet made for corrosives, with clear hazard labels, gives others a fair warning too, so surprises don’t happen mid-shift change.
Once, a minor leak left yellow streaks on a bench—this chemical loves leaving bright tracks. Wiping spills with a dry rag only spread the risk. Neutralizing spills with a careful layer of inert absorbent, using a scoop, and sealing waste in a safe bag, kept exposure in check. Rushed cleaning or shortcuts with water or acids can ramp up toxic vapors. Every cleanup effort deserves as much focus as the work itself.
Clear, direct training on what to do during spills helps everyone react with focus rather than panic. Eye wash stations, emergency showers, and clear walkways show value the day something goes wrong. Fast, confident response isn’t born in emergencies—it grows with short, repeated drills. Keeping emergency contacts posted right by the workspace, plus an action plan, fills in any gaps where stress might trip up memory.
Like any workplace, risks don’t always shout for attention. Chronic exposure to low levels of vapors links with lung and skin irritation, and there’s little room for gambling on health. Regular checks of workspaces and personal habits catch hazards before they snowball. Safety isn’t about big events; it’s in the habits you pick up and keep.
Sodium Benzothiazol-2-Yl Sulphide carries the formula C7H4NNaS2 and the CAS number 2492-87-7. Its core structure balances a benzothiazole ring with a sulfur-based functionality. The presence of sodium is more than just a technicality—this part of the molecule often decides how the compound behaves in water and interacts with other ingredients. You’ll find sodium benzothiazol-2-yl sulphide mostly as a pale yellow powder. Once it goes into solution, its chemistry unlocks a spectrum of uses, especially for the rubber industry.
The journey from raw chemical to practical value often runs through a mixing room lined with gloves and protective gear. I’ve seen engineers measure out this compound in bulk, ready to feed the demands of tire and gasket production. Sodium benzothiazol-2-yl sulphide acts as a vulcanization accelerator. The term might sound technical, but the real-world benefit means getting the right elastic bounce and long life out of a rubber product. If you put time on a production line, you start to appreciate that even small changes in additives like this one draw a hard line between reliable performance and breakdowns that cost serious money.
Opening a drum marked with the CAS 2492-87-7 brings a string of safety questions. The strong odor signals that this compound shouldn’t go airborne. Respiratory protection, gloves, and goggles move from recommendations to must-haves. Data from published research, including standard safety sheets, points to risks like skin irritation and breathing issues if handled carelessly. Storage guidelines focus on tight sealing and cool, dry spaces. I’ve watched teams run routine drills for handling spills and exposures, and there’s wisdom behind these habits: sharp protocols stop small mistakes from becoming serious incidents.
Sodium benzothiazol-2-Yl sulphide does not break down quickly in the environment. In countries with strong chemical management laws, reports trace its journey from factory waste to waterways. Research published in journals like Environmental Science & Technology shows how these residues linger in soil and water. If plants or fish collect the chemical, it can move up the food chain. Environmental managers I’ve worked with remind us that treating wastewater and capturing runoff is not negotiable. The science backs them up: local regulations set discharge limits for a reason, and testing regularly catches leaks before ecosystems pay the price. Failure to respect these systems steps right into legal and ethical problems.
Experimenting with substitute compounds sometimes lowers environmental risk—engineers try gentler chemicals when possible. Closed-loop production tech, which recycles water or captures emissions, cuts down on waste. Labs focus on tightening up analytical methods. Tracking even tiny amounts of sodium benzothiazol-2-yl sulphide can catch leaks early and prevent them from spreading. Training, not just for lab workers but for everyone in the plant, makes a clear difference in reducing mishaps. From experience, these hands-on solutions hold far more weight than just pushing around paperwork.
Demand keeps sodium benzothiazol-2-yl sulphide in play across continents. Every pound of it comes with responsibility. The right mix of chemical knowledge, hands-on safety, and strong environmental stewardship lines up with real-world quality—and with the standards set by both the law and public trust.
Sodium Benzothiazol-2-Yl Sulphide shows up in plenty of industrial processes. Over the years, in my work with chemical warehouses, I’ve seen the difference good handling can make. Let’s face it—if folks toss this stuff on a shelf, the risks shoot up, and the costs for mistakes add up fast. This isn’t hype; the safety data and decades of industrial experience have shown the dangers of skipping proper storage routines, especially with sulfide-based materials like this.
At room temperature, this chemical gives off an odor that clings to everything. Moisture in the room combines with it, leading to clumping or even hazardous decomposition. No one wants hydrogen sulfide gas drifting through the building. Even at low concentrations, hydrogen sulfide can lead to headaches, dizziness, and, at high exposure, it becomes fatal. I once saw a warehouse evacuation because a single drum leaked, so ignoring protocol isn’t just sloppy—it’s dangerous for workers and the environment.
The basics look like common sense, but they save lives. Strong containers that won’t corrode or break under the compound’s touch, tight lids that actually fit and seal, and labels that are clear and durable—simple stuff, but critical. Keep it away from acids and oxidizers. These combos cause reactions that release heat, gas, or both. A mistake with chemical separation once melted two metal shelves in a few minutes. Nobody enjoys cleaning up after a warehouse accident like that.
Many suppliers recommend keeping this chemical in a cool, dry area. In practice, that means shelves off the floor, away from damp spots. I’ve seen moisture sneak into unsealed drums in less than a week just because they sat near a loading dock door. A dry storage room pays off, especially with temperature controls. Even if the facility sits in a humid or tropical area, there are ways to keep humidity down—sealed floors, dehumidifiers, and regular inspections go a long way. Watch out for sunlight, too. Direct exposure breaks down the chemical faster, so a well-lit room matters less than keeping light indirect.
Ventilation turns from optional to non-negotiable. Fresh air cuts down on fumes, and good airflow prevents pockets of dangerous gas from building up. Routine air checks spot any problems early. Experience teaches workers to trust their noses and their personal monitors. Spills need catching trays underneath containers. In case of leaks, these trays stop the spread and make cleanup faster and safer. That’s one trick every facility should adopt.
Personal protective gear isn’t up for debate here. Gloves, goggles, and proper clothing block contact with the skin and eyes. People sometimes think a quick grab without gear won’t hurt, but the burns and rashes tell a different story. I’ve seen professionals forget, and they always regret it.
Storing Sodium Benzothiazol-2-Yl Sulphide safely keeps people healthy, protects investments, and keeps the environment free from contamination. It takes some up-front work—right storage containers, solid room controls, and dedication to the routine—but the benefit pays off every day. Risk isn’t a distant idea; it is real, and addressing it starts with what happens in the storeroom.
Sodium Benzothiazol-2-Yl Sulphide, often found in rubber production, plays an important part in speeding up vulcanization. Tire manufacturers, shoe factories, and similar industries lean on it heavily. Working in an industrial lab, I handled plenty of chemicals that needed extra safety routines, but this one always made me pause, even if protocols around it felt normal on the surface. I learned early to check runoff management before clocking out. Not every chemical feels risky at first glance, but the background data sometimes paints a different picture.
Once this substance serves its use in a factory, leftovers and waste can slip out into local waterways—unless someone pays close attention. Several studies show that this compound doesn’t just vanish. Water samples downstream from rubber plants reveal traces of benzothiazole sulfides, raising red flags among researchers. One peer-reviewed journal pointed out that even low concentrations can impair fish gill structure. My own time volunteering at a river cleanup taught me how persistent some chemicals are. Even after weeks of diligent work, the water held odd smells and left my hands dry, clear signs that something stubborn lingered beneath the surface.
Benzothiazole sulfides don’t break down quickly. Tiny creatures—crustaceans, insects, even worms—collect these particles in their bodies. Bigger fish feeding on them wind up at risk, and so do birds. Bioaccumulation doesn’t happen overnight, but once it gets started, stopping it becomes tough. I remember seeing fish deaths near an industrial site, later traced to chemical runoff. No one rushed to take responsibility at first, but environmental groups documented the chain reaction: pollution led to smaller food sources, weak fish, and upset local birds too.
People living near factories sometimes report odd tastes in well water or rashes after swimming. Studies haven’t finished tracing every possible pathway from chemical contact to illness, but there’s concern around skin and eye irritation, especially among factory workers. Careless handling or storage can spell trouble. I always wore gloves and goggles, even for a quick cleanup, because chemical labels often tell only half the story. Chronic problems sneak up slowly—and that’s what local advocacy groups worry about most. Nobody wants to discover five years later that a common chemical dumped in the creek caused lasting health issues.
Science alone doesn’t keep water clean. Plant managers need strong monitoring programs. Local government pushes for rules on chemical disposal help keep waste out of streams. In places where regulators test early and often, communities tend to spot problems sooner. Part of the solution involves updating machinery to recover or neutralize hazardous byproducts. The big lesson here comes from collaboration: factory workers, environmental scientists, and neighbors trading data, pushing for updates, meeting about weekly water test results. In my experience, conversations between these groups drive better habits and stronger rules.
Substitutes for sodium benzothiazol-2-yl sulphide exist, though switching takes investment and re-training. Forward-looking companies experiment with greener chemistry, seeing business value in staying ahead of environmental pressure. The transition may not feel convenient at first, but clean water and healthy wildlife show the results over time. My hope rests on seeing more industries taking these steps, guided by real-world outcomes rather than just rulebooks.
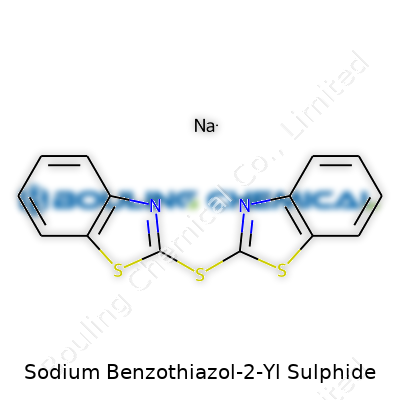
| Names | |
| Preferred IUPAC name | sodium 1,3-benzothiazol-2-ylsulfanylide |
| Other names |
2-Mercaptobenzothiazole sodium salt Sodium 2-mercaptobenzothiazole MBT-Na Sodium benzothiazole-2-thiolate |
| Pronunciation | /ˈsəʊdiəm ˌbɛnzoʊˌθaɪəzɑːl tuː aɪl ˈsʌlfaɪd/ |
| Identifiers | |
| CAS Number | 24948-91-8 |
| 3D model (JSmol) | `C1=CC2=NC(=S)SC2=C1.[Na+]` |
| Beilstein Reference | 529873 |
| ChEBI | CHEBI:87698 |
| ChEMBL | CHEMBL15829 |
| ChemSpider | 2301057 |
| DrugBank | DB11303 |
| ECHA InfoCard | 100.109.311 |
| EC Number | 294-506-1 |
| Gmelin Reference | 83296 |
| KEGG | C14494 |
| MeSH | D014059 |
| PubChem CID | 10954005 |
| RTECS number | DG6150000 |
| UNII | FQU637JJJO |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DJ7WQO5CU7 |
| Properties | |
| Chemical formula | C7H4NNaS2 |
| Molar mass | 199.27 g/mol |
| Appearance | Pale yellow to brown powder |
| Odor | Rubber-like |
| Density | 1.36 g/cm³ |
| Solubility in water | Soluble |
| log P | -1.8 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 3.5 |
| Basicity (pKb) | 7.83 |
| Magnetic susceptibility (χ) | -58.0e-6 cm³/mol |
| Refractive index (nD) | 1.630 |
| Viscosity | 80-120 cP |
| Dipole moment | 4.80 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 237.4 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | D08AX |
| Hazards | |
| Main hazards | Toxic if swallowed, causes skin irritation, causes serious eye irritation, may cause an allergic skin reaction, harmful to aquatic life with long lasting effects. |
| GHS labelling | GHS02, GHS07, GHS09 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P280, P301+P312, P302+P352, P305+P351+P338, P332+P313, P337+P313, P362 |
| NFPA 704 (fire diamond) | 2-2-0-Aside |
| Lethal dose or concentration | LD50 (oral, rat): 740 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 1950 mg/kg |
| NIOSH | SJ8575000 |
| PEL (Permissible) | 15 mg/m3 |
| REL (Recommended) | 5 mg/m³ |
| Related compounds | |
| Related compounds |
Benzothiazole 2-Mercaptobenzothiazole Sodium 2-mercaptobenzothiazole Zinc 2-mercaptobenzothiazole Diphenylguanidine |