Curiosity and necessity have a knack for shaping progress. Back in the early twentieth century, researchers began to dig deeper into the mechanics of amino acid metabolism. That’s where sodium 5-oxo-L-prolinate, often called sodium pyroglutamate, began showing up in lab notebooks and scientific journals. The discovery came as scientists tried to untangle the web connecting glutamic acid metabolism and neurological health. By the 1970s, as mental fatigue and cognitive support became talking points in both academia and pharmaceutical circles, production scaled up for clinical trials and new supplement lines. Its role in biochemical research, especially its involvement in the γ-glutamyl cycle, drove further studies and led to more refined syntheses. These days, demand comes from both the health industry and textbooks, reflecting how fundamental chemistry and real-life applications keep pace with each other.
Sodium 5-oxo-L-prolinate is a white crystalline powder, with a slight characteristic odor and a hint of salty taste—traits familiar to anyone who’s handled amino acid salts. This substance dissolves readily in water, leaves little residue, and has become a workhorse in processes that call for stability and consistent composition. Bulk producers pay close attention to the details of purity, not just for safety, but to ensure it performs consistently in formulations aimed at pharmaceuticals, cosmetics, and functional foods. Purity levels above 98% now come standard, signaling a maturity in manufacturing practices. Packaging gets robust treatment to shield against light and moisture, as this material draws humidity from the air, clumping if left exposed.
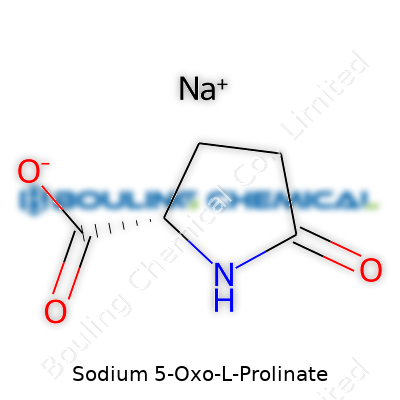
Chemically, the structure comes down to the sodium salt of 5-oxo-L-proline, putting it among the cyclic derivatives of glutamic acid. Its molecular formula is C5H6NNaO3, and it tips the scales with a molecular weight of 153.09 g/mol. The physical side tells a story of fast solubility in water—important for both oral and topical uses—while it hardly dissolves at all in ethanol or most organic solvents. Melting point measures sit between 180–188°C, but practical handling never reaches those temperatures, keeping degradation to a minimum. The pH of a 5% solution lands near 7.5, which keeps it gentle for skin contact and easy on biologically sensitive mixtures. With no strong odor or off-color, it slips easily into product lines where visual and olfactory neutrality count.
In manufacturing, technical specifications become the daily language of chemists and quality control experts alike. Particle size ranges from fine (60–100 mesh) to ultra-fine, fit for precise blending operations. Moisture content holds below 1%, so the powder handles predictably and resists caking. Heavy metal residues, important due to increasing global safety standards, stick well below regulatory thresholds—typically under 10 ppm for lead. Most suppliers provide documentation, such as Certificates of Analysis, specifying batch numbers, country of origin, and shelf life, which normally tops out at two years in sealed containers. Labeling requirements ask for unambiguous naming: sodium 5-oxo-L-prolinate, sodium pyroglutamate, or the relevant E-number for food use, along with comprehensive handling, storage, and hazard symbols as required by global transport authorities and local health commissions.
Industrial chemists favor routes that pair efficiency and safety. Synthesis starts with L-glutamic acid, which reacts under mild cyclization conditions—usually with the help of heat—to form L-pyroglutamic acid. Neutralization by sodium carbonate or sodium hydroxide produces the desired sodium salt. Purification steps mainly involve filtration, controlled crystallization, and repeated washing to shed by-products. Water stays as the solvent of choice for most steps—safer for workers, easier on disposal systems, and a nod to sustainability efforts. Advances in automation and real-time monitoring have shaved reaction times, increased yields, and produced a product with tighter particle size and purity distributions than just a decade ago.
Chemically speaking, sodium 5-oxo-L-prolinate behaves as a stable substrate under most ambient conditions. Oxidation occurs only under harsh environments—strong oxidizers at elevated temperatures, a rare event in most production setups. Reductive openings of the ring structure remain a hot topic for researchers looking at derivatives for use as enzyme substrates or as chemical intermediates. The carboxyl group undergoes standard reactions found in amino acid chemistry, such as esterification or amidation, providing a springboard for new compound development. Innovative chemists in pharmaceutical R&D often use mild catalysts or biologically inspired enzymes to forge modifications that tune solubility or target specific metabolic pathways.
Familiarity breeds nicknames, and this compound has plenty. Sodium pyroglutamate serves as the most common English label, but chemists also recognize terms like sodium pidolate, sodium pyrrolidone carboxylate, and E370a in food ingredient lists. In scientific circles, it’s not uncommon to find references such as L-pyroglutamic acid sodium salt or natrium 5-oxo-L-prolinat. Brand names crop up in cosmetic and nutraceutical spaces, reflecting a drive to pique curiosity and promise benefits that trace back to that simple five-membered ring at the core.
Regulatory bodies pay keen attention to worker and consumer safety. Material Safety Data Sheets recommend gloves and goggles for operators, mostly as a matter of best practice due to the fine dust. Eye contact or inhalation can cause irritation, yet ingestion in normal nutritional supplement or food amounts stays within accepted risk thresholds. Storage in airtight containers away from strong acids or bases reduces unwanted product changes or corrosion of storage vessels. Disposal instructions follow local environmental protections; sodium-based waste stays relatively benign but enters approved municipal treatment to keep everything above board. Modern manufacturers supply detailed traceability reports, supporting the growing trend for ingredient transparency in consumer products, and those reports get reviewed at audits and during international customs inspections.
Sodium 5-oxo-L-prolinate finds a place where function meets formulation. In cosmetics, it acts as a humectant, drawing moisture and helping skin products deliver a soft, non-greasy finish. Personal care brands use its mild nature and compatibility with sensitive skin as a selling point. Nutrition companies blend it into supplements, aiming to improve cognitive performance, reduce mental fatigue, or support stress resilience. In pharmaceuticals, it stands as an ingredient in products designed for cognitive function or as a stabilizer for active ingredients. Food scientists explore its umami-enhancing properties and as a mineral source that integrates without fuss into diverse recipes. Agriculture and animal husbandry consider it as a metabolic supplement, with studies underway to gauge its effect on feed conversion and animal well-being.
Scientific curiosity keeps sodium 5-oxo-L-prolinate in the sights of many research teams. Neuroscientists devote lab hours to mapping its effects on neurotransmitter cycles, especially where glutamate and GABA balance influences mood and cognition. Pharmacologists assess whether the compound can improve efficacy in drug delivery by stabilizing sensitive actives or boosting bioavailability. Analytical chemists refine detection methods, developing new assays that pin down trace components in complex formulations. Cosmetic chemists test combinations with other skin-benefiting amino acids, searching for ways to extend hydration or reduce irritation in finished products. Collaborations with universities and contract research organizations open new doors, especially for incremental improvements in manufacturing agility, purity, and green process chemistry.
Long-term safety matters, so toxicologists have committed years to evaluating dosing limits and potential side effects. Current literature points to a generally low toxicity profile in both animal models and human studies, assuming regular use amounts. High-dose regimens rarely provoke adverse reactions, though lab animals exposed to chronic, supra-nutritional doses have shown mild renal or hepatic changes. Regulatory reviews ask manufacturers to report detailed toxicology panels, from acute oral and dermal toxicity to genotoxicity screens and reproductive safety. Because sodium 5-oxo-L-prolinate appears in dietary supplements, some oversight stays stricter than for pure food additives or industrial chemicals. Academic researchers continue to monitor for allergic responses or rare metabolic sensitivities, but modern consensus places this compound among the safer amino acid derivatives in regular commerce.
New discoveries rarely finish at the lab bench—commercial appeal and humanitarian goals tug technology in new directions. Sodium 5-oxo-L-prolinate enters the stage at a vital moment; consumers crave gentle, bio-compatible ingredients, and industry leaders look for dual-use molecules that work just as well in nutrition as in skincare formulations. Emerging research hints at more subtle roles in cognitive support, neuromodulation, and perhaps as a nutraceutical partner to prescription therapies. Production methods shifting towards green chemistry—lower energy, fewer solvents, smarter catalysis—will meet the social push for sustainability. With brain health topping healthcare priorities and global food chains leaning toward value-enhanced fortification, sodium 5-oxo-L-prolinate stands ready for a new round of research, scaling, and public attention. Those who push boundaries and ask the tough questions will have the biggest say in what the next chapter brings, driven by the lessons learned from chemistry textbooks, factory floors, and the people who use these formulations every day.
Sodium 5-Oxo-L-Prolinate doesn’t sound friendly at first. The name alone can scare folks away from checking what it does. Still, you’ll spot this ingredient in places where it gets far less attention than it deserves. Behind that long name sits a salt derived from an amino acid, and for folks who keep an eye on nutrition or manufacturing, this ingredient has real value.
Anyone who picks up processed snacks or nutritional shakes might have consumed this compound. Technically, it comes from proline, an amino acid our bodies use all day long. Food makers add it for a couple of reasons. It acts as a stabilizer—helping powdery mixes behave themselves and stay mixed instead of clumping. It also plays a part as a flavor enhancer, giving savory notes or helping round out sharp tastes in soups, broths, or protein bars. A Japanese study from a few years back tested its impact on taste and found it could actually enhance umami flavors.
I’ve worked in kitchens and seen firsthand how small tweaks to additives change the way foods hold up on the shelf. Ingredients like this one aren’t flashy, but without them, shelf-stable shakes or sports drinks often taste off or spoil before you finish the bottle. One small tweak in the mix and you get something that lasts longer and tastes better.
Drug makers pay attention to sodium 5-oxo-L-prolinate for another reason. In some medication formulas, it acts as a buffering agent, keeping pills and powders stable inside their packaging. In the body, it breaks down into natural building blocks—nothing harmful—so scientists don’t worry about it sticking around and causing trouble. It’s sometimes found in solutions used for patients needing extra hydration or in special IV fluids for people with kidney disease or metabolic problems.
I’ve seen debates between clinicians over which additives really matter for patient safety. While some worry about any extra chemicals, research so far shows no evidence this one brings harm. In fact, doctors in hospitals sometimes rely on its ability to help regulate acid-base balance in tricky cases.
Data from animal studies points toward a possible brain benefit. Sodium 5-oxo-L-prolinate participates in a metabolic system that feeds into glutathione production. Glutathione is a key antioxidant that the brain uses to protect itself against damage from stress and pollution. No magic bullet for memory loss or dementia, but researchers keep circling back to this pathway as a potential target for health supplements.
Of course, more double-blind human trials would settle whether regular supplements help with mood or memory. Still, the fact that scientists keep studying it means enough promising results have been seen. Nutraceutical makers watch this research and adjust their supplement blends based on what gets published.
With any ingredient showing up in food and pharmaceuticals, attention on safety and impact is constant. Regulators look for long-term studies, and responsible manufacturers need open lines for reporting side effects or allergic reactions. A safe record today doesn't promise tomorrow’s products don’t go off track, so transparency stays critical. Companies that provide evidence of testing and traceability win trust. As consumers, asking questions about labels and ingredient origins is always the smart way forward.
Sodium 5-oxo-L-prolinate probably won’t take over Instagram feeds or TikTok trends. But in industries that bet on stability, taste, and safety, it quietly gets the job done.
A lot of scientific jargon can make ingredient lists feel like a puzzle. Sodium 5-oxo-L-prolinate sounds daunting on a label, but it’s a salt form of an amino acid derivative related to proline, which already occurs in the body and in plants. Folks who read skincare science know that these related amino acids often help skin stay hydrated and recover from stress.
This salt acts as a humectant. That means it keeps moisture locked in the skin. People with dry skin or those who use lots of cleansers appreciate ingredients that help repair what gets stripped away. I’ve seen this ingredient in lightweight serums and soothing creams. Compared to heavier occlusives, it feels like nothing at all but still calms tightness in my skin after showering.
More than just anecdotal, lab data supports the value of small, water-attracting amino acids. Sodium 5-oxo-L-prolinate pulls water from deeper layers of skin and from the environment, so skin feels soft longer. One study from a Japanese cosmetics group found that formulas with this ingredient improved skin elasticity in a matter of weeks. In daily life, anything that keeps skin’s barrier healthy stands to cut down irritation and flakes.
In safety circles, this ingredient hasn’t triggered red flags. Regulatory groups in North America, the EU, and Japan have checked data and judged it safe for use in rinsed and leave-on skin products. The Cosmetic Ingredient Review (CIR) panel dug through animal and human tests—no allergic reactions or skin cell toxicity came up at the concentrations found in products. The European SCCS reported the same for patch tests and long-term use on volunteers.
Anyone with super sensitive skin knows that even “safe” ingredients can sting. If you have eczema, or have had trouble with any amino acid derivatives before, a patch test behind the ear for a few days could help. But for most folks, research doesn’t show much risk. It’s not like preservatives or fragrance ingredients that can mess with a lot of people.
People want to know what they put on their skin. Ingredient overload has trained a generation to scrutinize what goes on the face and arms. I often cross-check every new label, especially when my skin’s acting up. Science-backed transparency built trust in brands, and consistent results built my loyalty to the formulas that clearly disclose every component.
Rising allergy rates, hard water, and urban pollution made people more thoughtful about daily skincare. Sodium 5-oxo-L-prolinate doesn’t have the baggage of old-school irritants. Still, brands and formulators have a job to do: keep researching, watch for rare reactions, and adjust concentrations in children’s or hyper-sensitive products. Dermatologists and pharmacists should help people match the right science-backed ingredients to individual needs, not just follow the latest buzz.
Open dialog between brands, big lab groups, and real-world users will help push for safer skincare. When customers ask about ingredients like sodium 5-oxo-L-prolinate, brands should share research or data, not just recycle sales claims. With that kind of give-and-take, the industry can stay grounded in the science, and consumers can trust their choices a little more.
Sodium 5-oxo-L-prolinate, more commonly known as sodium pyroglutamate or sodium PCA, shows up on ingredient lists for a reason. It does a lot more than sound impressive—it helps keep skin moisturized and feeling comfortable, especially in leave-on products. Many skin experts refer to it as a natural moisturizing factor mimic, owing to its close ties with the compounds our skin produces to retain water. Anyone who struggles with dry skin in the winter knows just how valuable moisture-retaining ingredients can feel after a hot shower.
To answer the question about concentration, most formulators land between 0.2% and 4% for topical use. This range comes directly from independent safety reviews and global regulatory bodies such as the CIR (Cosmetic Ingredient Review) and the European Commission. Both point to an upper limit of 4% for leave-on products, which brings a high level of comfort, especially for consumers with sensitive skin or barrier issues. Going above this range tends to risk irritation, which defeats the purpose entirely.
It’s tempting to think if a little bit works, then more must work even better. That idea rarely pays off in skincare. Sodium 5-oxo-L-prolinate acts as a humectant, pulling moisture from the environment and drawing it into the upper skin layers. Used sparingly, it helps support suppleness and elasticity. Pushing concentration higher than necessary can pull too much water, leading to irritation or even discomfort, especially in dry climates where there’s not much moisture in the air to grab onto.
Personal experience shows that using products with sodium PCA at around 2% leaves skin feeling noticeably plumper. Those with oily skin types might benefit from lower levels, around 0.5%, to avoid a tacky finish. Users tackling dehydration or persistent tightness could look for 3-4% in rich serums and creams. Dermatologists often recommend patch testing for new products, regardless of concentration, to head off any surprise sensitivities.
Diving into published literature, sodium 5-oxo-L-prolinate’s safety profile looks strong within the recommended range. The CIR’s expert panel reviewed studies on cumulative irritancy and concluded that concentrations up to 4% in leave-on products gave no systemic concerns and little risk of rash or redness in healthy volunteers. Scientific research from Japan highlights that even long-term use at 2% rarely produces irritation.
Users value trust, so companies need to share concentration information transparently. Labeling won’t always show exact percentages, but marketing teams and formulators should work together to communicate when a formula hovers near the upper limit. Brands committed to Google’s E-E-A-T principles—showing real-world experience, sourcing findings, and helping users make informed picks—build stronger loyalty.
Keeping sodium 5-oxo-L-prolinate within 0.2-4% protects skin and delivers real benefits. Formulators juggle interactions with other actives, stability in changing weather, and the diversity of skin types. As consumers ask more questions, manufacturers face pressure to base included concentrations on clinical evidence, not just marketing trends. Following published safety benchmarks and personal testing, cosmetic companies set a precedent for responsibility, helping shoppers feel secure about what they’re putting on their faces.
These days, folks check labels more than ever. Whether an ingredient comes from a dietary supplement, pharmaceutical recipe, or the latest skincare formula, each name on that tiny print means something. Sodium 5-Oxo-L-Prolinate pops up across a few of these settings, promising various benefits—mainly as a moisturizer or, in some cases, metabolic aid. Yet, like most additives, it isn’t immune from drawing concern about safety or side effects.
Of course, years of research let us spot trouble before it escalates. In medicine, experiences count. Take my time working alongside pharmacists—questions about sodium compounds show up all the time. With this one, most people glide through fine. At standard doses in topical or oral products, adverse reactions turn out to be genuinely rare, especially compared to harsher ingredients.
Yet, every person’s body works differently. A handful will get redness, mild itching, or even a stinging feeling if sensitive skin comes in contact with the compound. If you’re using something new, this isn’t a surprise. Any product, even one marked as “hypoallergenic,” can cause irritation. Reports of these problems remain uncommon, but not nonexistent.
Some websites list headaches or stomach upset linked to oral supplements that contain sodium 5-oxo-L-prolinate, often because folks exceed recommended intake. In higher doses—much more than found in a moisturizer or fortified drink—too much sodium can add strain to kidneys, or in rare situations, raise blood pressure. People with chronic kidney problems or anyone on a sodium-restricted plan should always ask a doctor before picking up a supplement that adds to daily sodium load.
Trust grows once you see transparency from brands and manufacturers. For me, that means reading ingredient amounts, studying voluntary recalls, or scanning databases like PubChem for safety research. Putting in this effort matters, especially if you’ve got allergies, compromised skin, or you live with conditions that turn tiny risks into big ones.
Clear labeling helps a lot. Reputable brands follow legal limits and update packaging with information on potential skin irritation, overdose risks, or how the additive works with other ingredients. This is more than just covering their bases—it gives buyers real power to make safer choices. The Gold Standard for me will never be trend-driven promises, but brands leaning on peer-reviewed science and honest warnings.
A patch test offers a small dose of peace of mind. Applying a tiny amount of a product to the inside of your arm and waiting a day or two cuts down risk, especially for people prone to sensitivities. If you feel burning or itching, stop right there. Those with health concerns—like kidney or heart issues—should bring the supplement bottle to their next appointment and make space for a real chat about safety.
Education beats guesswork every day of the week. Reaching out to pharmacists brings fast, tailored advice, especially for anyone managing complex prescriptions. My own experience: a quick call or short in-person conversation heads off side effects, interactions, or waste. At the end of the day, being deliberate beats blind faith in claims on the front of any bottle.
Sodium 5-Oxo-L-Prolinate looks safe for most people, especially at standard levels and when formulas are used as designed. Of course, no ingredient earns a universal free pass. Being aware, asking good questions, and reviewing sources—these three steps land you in a much safer spot, regardless of the latest health trend.
Sodium 5-oxo-L-prolinate often appears on ingredient lists for moisturizers and serums claiming to soothe or hydrate. This ingredient, sometimes known as sodium pyroglutamate, functions as a humectant. By drawing water into the upper layers of the skin, it helps keep things looking plump and calm, which many folks with sensitive skin appreciate.
Plenty of people, myself included, know the struggle of skin getting red or itchy from even basic products. One winter, my cheeks flared up just from changing laundry detergent. I started checking every product label, looking for language around non-irritating or "safe for sensitive skin." The technical names on those lists often felt intimidating.
Sodium 5-oxo-L-prolinate stood out for its background. Dermatologists and well-respected research suggest this stuff closely mimics the natural moisturizing factors found in our own skin. Scientific studies—including data published in journals like the International Journal of Cosmetic Science—report that this ingredient really does help keep moisture from slipping out of the skin, especially in dry climates or after washing.
Products formulated for sensitive skin benefit from humectants that don’t sting or cause a tight feeling. Testing from independent safety panels, including data reviewed by the CIR Expert Panel, shows sodium 5-oxo-L-prolinate hardly ever triggers reactions, even for people with delicate skin. The ingredient gets used at low levels, usually between 1% to 5%, providing enough hydration without becoming overwhelming.
Other humectants—like glycerin or hyaluronic acid—sometimes leave a sticky film that isn't comfortable for everyone. Sodium 5-oxo-L-prolinate tends to absorb better, often leaving skin smooth and soft, based on user studies. Those who are sensitive to fragrances or other additives will find a lot of sodium 5-oxo-L-prolinate formulas being fragrance-free, too.
Trust often comes down to a brand’s transparency. Many companies making claims about soothing ingredients now share allergy test data and clinical trial results on their websites, and this sort of honesty matters. Reviews by organizations like the Environmental Working Group give sodium 5-oxo-L-prolinate a green light for non-toxicity.
Still, individual sensitivities can differ. Anyone who deals with eczema, rosacea, or even unpredictable flare-ups probably checks labels closely. My routine today includes a cream with sodium 5-oxo-L-prolinate; it never set off my usual burning or redness, even after a long day outside or after a retinoid treatment.
Education leads to better picks. Relying on a single "hypoallergenic" claim isn’t enough. Digging into research, asking dermatologists, and patch testing new products all help keep skin calm. Look for specific information—clinical data, allergy testing, inclusion at low and safe concentrations—before trying something new.
For those navigating the maze of sensitive skin, sodium 5-oxo-L-prolinate stands out as a strong ally. With decades of research, responsible use in well-made products, and support from experts, it fits into smart routines without much worry. People deserve hydration without side effects, and this ingredient makes that possible.
| Names | |
| Preferred IUPAC name | Sodium (2S)-5-oxopyrrolidine-2-carboxylate |
| Other names |
Sodium pyroglutamate Sodium pidolate Sodium 5-oxopyrrolidine-2-carboxylate |
| Pronunciation | /ˈsəʊdiəm faɪv ˈɒk.səʊ ɛl proʊˈlɪ.neɪt/ |
| Identifiers | |
| CAS Number | 6610-41-5 |
| 3D model (JSmol) | `3D model (JSmol)` string for **Sodium 5-Oxo-L-Prolinate** (also known as Sodium L-pyroglutamate) in **JSmol**: ``` [NH3+][C@@H](CCC(=O)[O-])C(=O)[O-].[Na+] ``` *This is the SMILES string suitable for 3D visualization in JSmol.* |
| Beilstein Reference | 113754 |
| ChEBI | CHEBI:87681 |
| ChEMBL | CHEMBL3426589 |
| ChemSpider | 22134704 |
| DrugBank | DB14634 |
| ECHA InfoCard | 03-2119980989-37-0000 |
| EC Number | 24694-55-7 |
| Gmelin Reference | 113159 |
| KEGG | C01879 |
| MeSH | D015308 |
| PubChem CID | 23727860 |
| RTECS number | TC5950000 |
| UNII | F4X58J6B14 |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID00940798 |
| Properties | |
| Chemical formula | C5H6NNaO3 |
| Molar mass | 173.12 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 1.477 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.8 |
| Acidity (pKa) | 2.45 (at 25 °C) |
| Basicity (pKb) | 7.40 |
| Magnetic susceptibility (χ) | -42.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.485 |
| Dipole moment | 9.5407 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 344.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −870.0 kJ/mol |
| Pharmacology | |
| ATC code | N06BX13 |
| Hazards | |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | > 100 °C |
| LD50 (median dose) | LD50 (median dose): > 2000 mg/kg (oral, rat) |
| PEL (Permissible) | Not established |
| REL (Recommended) | 5 g/L |
| Related compounds | |
| Related compounds |
Proline L-Proline 5-Oxo-L-proline Pyrrolidone carboxylic acid Sodium L-prolinate |