Industries first became interested in Sodium 5-Oxo-Dl-Prolinate as researchers started digging into the cyclic forms of amino acid derivatives in the mid-20th century. Scientific curiosity about proline derivatives and their salt forms sparked the earliest patents, and labs exploring ways to stabilize compounds with active carboxyl or amine groups soon came across this sodium salt. Early work often centered on the compound’s role as a model system for peptide research. Since then, sodium 5-oxo-DL-prolinate has steadily found new homes in different fields—both in theory and in practice. The path from bench-scale synthesis to inclusion in product libraries shows how this compound found respect in chemical and biotech circles.
Sodium 5-Oxo-Dl-Prolinate comes in crystalline or powder form. The compound serves as a functional building block for various chemical syntheses, admired for its stable nature and compatibility in aqueous and non-aqueous systems. Producers of peptide intermediates, pharmaceutical research teams, and engineers working on biodegradable chelating agents regularly turn to this salt. Its popularity stems from its clear performance in technical applications, including its role as a reactant and process aid.
This compound carries a molecular formula of C5H6NNaO3, a molecular weight around 155.1 g/mol, and presents as a white or off-white solid. Sodium 5-oxo-DL-prolinate dissolves easily in water, offering a neutral to slightly basic solution, thanks to the sodium ion. Its melting point sits above 200°C (decomposition) and it remains fairly stable under typical laboratory conditions. Chemical structure modeling shows a five-membered lactam ring, which resists simple hydrolysis and stands up to short bursts of high pH without breaking down. All these features mark the compound as a practical intermediate for chemists who need reliability batch after batch.
Manufacturers test for purity above 98%, verified using chromatography or titration. Impurity profiles include limits for proline, sodium chloride, and other close relatives. Containers ship labeled with CAS number 7704-72-5, product code, batch number, net weight, and safety information such as hazard symbols for skin and eye irritation based on classification. Safety Data Sheets accompany each shipment. I’ve seen plenty of suppliers meeting up with European, US Pharmacopeial, and REACH standards, especially for research or pilot plant quantities. The details, including water content by Karl Fischer and particle sizing, turn out crucial when researchers aim for scale-up.
Industrial production often starts from 5-oxoproline (also called pyroglutamic acid), with direct neutralization by sodium hydroxide in water, followed by careful crystallization and drying. I’ve spoken to technicians who highlight the need for temperature control during crystallization to avoid co-precipitation with sodium chloride, which affects downstream purity. Filtration and drying processes may run under vacuum to prevent hydrolysis or racemization. Labs may adapt this process—for example, choosing lyophilization for smaller, more sensitive batches. Firms moving toward greener chemistry try to minimize solvent or energy use, sometimes integrating in-line purification to reduce waste.
As a five-membered lactam sodium salt, it participates readily in nucleophilic substitutions, ring-opening reactions, and amidation. Researchers have turned to sodium 5-oxo-DL-prolinate to build more complex peptidomimetics, modify surfaces for biomedical devices, or test enzyme activity with cyclic substrates. Functionalization at the alpha-carbon allows for the creation of novel derivatives—advancing fields as diverse as human health and polymer development. Concerns about side reactions—for example, epimerization or decarboxylation—crop up, yet careful reaction monitoring keeps the process on track for most users. Chemists appreciate its balance of reactivity and predictability.
Sodium pyroglutamate, sodium 5-oxo-L-prolinate (or DL-prolinate for the racemic mixture), and sodium PID—these crop up in literature and product sheets. Sigma-Aldrich, Tokyo Chemical Industry, and other chemical distributors offer it under proprietary names, generally cross-referenced to the CAS number. Some suppliers shorten the name to Na-pyroglutamate or use foreign language equivalents, leading to a patchwork of synonyms depending on region and supplier. Users often double-check labeling to prevent mix-ups, especially when quality matters.
Handling runs under standard chemical hygiene rules: gloves and eye protection, good ventilation, and prompt cleanup if spills occur. MSDS sheets point out minor skin and eye irritation but generally no systemic toxicity at expected exposure levels—although chronic effects deserve more attention. Workers avoid inhalation of dust, and storage happens in well-sealed containers, away from moisture and direct sunlight. Disposal adheres to local environmental regulations, typically involving dilution and neutralization before release. As someone who’s seen accidents from poor labeling, I can’t overstate the value of clear hazard communication and routine training on spill response.
This compound shows up in diverse environments: peptide synthesis, pharmaceutical intermediates, cosmetics, and food ingredient research. Scientists studying glutamatergic neurotransmission use sodium 5-oxo-DL-prolinate as a model substrate in enzymatic assays. In the cosmetics industry, it features as a humectant and skin-conditioning agent, taking advantage of its affinity for water and skin. Food technologists keep it handy when researching flavor enhancers, while plant biologists explore its function as a stress modulator in crop science. The sheer range of applications reflects both the compound’s chemical stability and biological compatibility.
R&D labs keep finding ways to tune derivatives—shifting from academic curiosity to real-world solutions. Recent work centers on modified versions for targeted drug delivery, given the compound’s ability to blend into biological pathways. Some projects aim to attach therapeutic agents or imaging markers to the lactam ring, improving selectivity and reducing adverse reactions. Efforts to boost process greenness, like biocatalytic production from renewable sources, gain traction as regulatory pressure mounts. Now and then, interdisciplinary teams combine physical chemistry, pharmacology, and engineering to open new avenues—each project a bet on the compound’s untapped potential.
Studies so far suggest low toxicity in mammals, both in acute and chronic settings, which explains why regulatory agencies show limited concern at standard industrial concentrations. Oral and dermal LD50 figures land comfortably high in rodents, but gaps in knowledge remain, especially with respect to long-term exposure and environmental buildup. Scientists call for better epidemiological data and more nuanced models of how sodium 5-oxo-DL-prolinate behaves in soil and water. Environmental researchers track its degradation for any trace-level impact on groundwater or aquatic systems—not just out of caution, but out of lessons learned from past chemical releases.
As the life sciences push for more sustainable ingredients and greener manufacturing, demand for versatile, low-toxicity chemicals like sodium 5-oxo-DL-prolinate won’t slow down. The compound’s use as a chemical precursor gives it staying power in pharma and agriculture. Next-generation synthesis routes—perhaps enzyme-catalyzed or powered by renewable energy—could reshape its production economics. If researchers unlock more about its role as a biochemical modulator, sodium 5-oxo-DL-prolinate could show up in more functional foods or medical devices. As a chemical I’ve encountered both on the benchtop and in large-scale setups, it stands as a quiet, reliable performer ready for whatever challenges industry and science send next.
Stepping into a pharmacy or scanning the back of a skin cream, you might notice words you’ve never even tried to pronounce. Sodium 5-oxo-DL-prolinate is one of those. Its complex name hides a fairly simple purpose. This compound turns up in more corners of daily life than most folks realize – not just in skincare but also in medicine and food.
Plenty of skincare products highlight moisture, promising plump, glowing skin. Behind some of these claims is sodium 5-oxo-DL-prolinate. This ingredient pulls moisture from the air, helping lock it into the skin. It plays a role much like the natural moisturizers found in healthy skin already. I’ve used creams with this stuff and noticed fewer dry patches in dry winter months. Dermatologists agree; products with this ingredient can help keep skin healthy, especially when used as part of a broader routine with sunscreen and gentle cleansers.
What gives it credibility? Research supports the ingredient’s ability to support the skin’s barrier and hold water. That’s important for people facing dryness from cold weather, harsh soaps, or conditions like eczema. It isn’t a miracle worker, but it adds real value as one step in better skin care.
Some medicines use sodium 5-oxo-DL-prolinate for its stabilizing properties. It shows up as an excipient—a helper that ensures a pill or cream doesn’t fall apart before reaching its target. Drug manufacturers lean on it for this reliability. The U.S. Food and Drug Administration permits its use because it’s safe at low doses, and toxicologists have set clear limits. Medicine gains shelf life and predictable delivery, benefiting patients who need accuracy in their treatment.
This ingredient slips quietly into food processing, too. Some processed foods include it for its protein-building potential. It can help stabilize proteins and keep food textures appealing, especially after long storage. In sports nutrition drinks or bars, it helps round out amino acid profiles. That’s handy if someone can’t get certain nutrients from regular eating. Some formulas for hospital nutrition use it to improve protein content for patients struggling to eat enough. Evidence shows it can help body tissue bounce back in recovery, as it fits smoothly into the body’s own metabolic cycles.
Not everything with a long name brings risks. Most reputable studies show this compound carries low toxicity and is well-tolerated. Allergies are rare. Experts do watch how much gets added to foods or creams, keeping amounts below known safety thresholds. In my own reading, it’s clear that reputable manufacturers and regulatory watchdogs take these safeguards seriously. Still, the industry benefits from transparency and clear labeling, so consumers know what’s ending up in their bodies and on their skin.
People don’t always realize how science sneaks into their morning routine or their lunch. Sodium 5-oxo-DL-prolinate stands as a reminder to look a little closer at ingredient lists. Consumers can make smarter choices by checking facts, leaning on independent research, and asking healthcare providers for guidance. If more people took a minute to explore these labels, there might be fewer surprises and more trust in the products we all reach for every day.
Sodium 5-oxo-DL-prolinate turns up in ingredient lists for many skincare products, especially moisturizers and serums promising better hydration. This ingredient joins amino acids and small-molecule humectants that draw water into the top layers of skin. As someone who has sensitive skin and spends quite a bit of time reading fine print on bottles, I watch for claims that make bold promises. The science behind this ingredient comes from its relationship to proline, a building block that helps skin stay plump and supported.
Research doesn’t treat every ingredient equally. Not every new compound gets dozens of long-term clinical trials. For sodium 5-oxo-DL-prolinate, safety data often comes from in vitro studies and short-term patch tests in small groups of people. Those studies usually show that it behaves much like other amino acid salts—mild, non-irritating, and not likely to trigger allergies in most users. Cosmetic safety organizations in Europe and North America have reviewed it. The Cosmetic Ingredient Review (CIR) assessed its structure and metabolism, finding no signals of toxicity at concentrations used in creams and lotions.
Personal care brands use this ingredient at rates around 0.2% to 2%—levels much lower than those needed to cause redness or skin barrier problems in volunteers during trials. At the same time, everyone’s skin acts differently. People who struggle with eczema, rosacea, or frequent reactions should test a small amount before using new products widely. This is standard advice for any skincare innovation. One thing I wish more companies did: post their research or in-use test results somewhere shoppers don’t have to dig.
Most people just want to know if something helps or hurts. Based on what I’ve read and tried, sodium 5-oxo-DL-prolinate helps skin grip moisture without leaving sticky residue. Skin feels softer and looks smoother, especially during winter or after sun exposure. Some brands market it to rival hyaluronic acid or urea, other favorites for hydration. No surprise, results depend on the product’s full recipe and the other ingredients that support or lessen its effects.
Problems may appear in rare cases. If skin stings, turns red, or starts peeling, stop using any product with this or a similar molecule. These reactions rarely trace back to just one ingredient, but keeping a list of triggers speeds up the detective work with dermatologists. Sometimes reactions result from scented compounds, preservatives, or another less-publicized part of the formula rather than sodium 5-oxo-DL-prolinate itself.
More transparency always helps. Posting safety test protocols and summary results helps buyers judge for themselves. Full ingredient lists make it easier for people with allergies or skin sensitivities. Some companies already do this, but the beauty industry as a whole could stand to raise its standards. Independent lab testing, carried out by organizations with no ties to the manufacturer, gives shoppers an extra layer of trust.
Honest marketing counts for more than glossy ads. Instead of vague claims, brands should describe how an ingredient works, what results are reasonable, and who should be careful. From my experience, talking plainly with customers builds better loyalty than promising a miracle in a bottle.
Patch testing tops my list before adding anything new to a routine—especially if your skin throws tantrums from time to time. Choose fragrance-free versions if allergies run in your family. Hold on to unused packaging until you know a product suits you. Save photos of ingredient lists for future reference.
Science points to sodium 5-oxo-DL-prolinate as a generally low-risk choice for most people hunting for moisture support. Still, no single solution works for all, and keeping skin healthy often comes down to paying attention, reading labels, and not falling for hype.
Sodium 5-Oxo-Dl-Prolinate doesn’t show up in daily life for most people, but it matters a lot for researchers and businesses working with specialty chemicals. In any lab, the safety of team members and the quality of results rest on careful storage. Over my years working alongside scientists and logistics coordinators, it’s clear that cutting corners—especially with less-common chemicals—leads to frustrating setbacks and sometimes real dangers. Sodium 5-Oxo-Dl-Prolinate deserves the same respect.
Exposure to moisture, wide swings in temperature, or contact with the wrong substances can alter the integrity of Sodium 5-Oxo-Dl-Prolinate. Water can encourage it to clump, degrade, or foster microbial growth (rare, but possible). As anyone who has watched a low-grade powder turn into a sticky mess knows, moisture protection isn’t just about meeting guidelines. It guards your work and often protects thousands of dollars of investment in materials, equipment, and human hours.
Heat is another enemy. At high temperatures, many sodium-based compounds break down or react in unexpected ways. So, scientists stick with cool, dry areas, protected from sunlight. Common sense tells you: if it feels muggy or warm inside the storage room, try again.
After conversations with academic lab managers and a stint volunteering at a small biotech startup, some storage guidelines rise to the surface:
Caring for Sodium 5-Oxo-Dl-Prolinate means keeping an eye on temperature controls, humidity, and the separation from incompatible materials. Teams put in the extra effort not just for compliance, but for the confidence that comes with knowing every bottle is as pure as the day it arrived. The best lessons often come from a single ruined experiment—after that, nobody ever skips the desiccant again.
Respected sources, including the Sigma-Aldrich catalog and several safety data sheets, back up this approach. A little vigilance in storage preserves chemical quality and makes complex research possible. Safe, dry, and away from heat—those three ideas create a foundation for chemical handling success.
Food additives spark a lot of debate. Every new entry on the ingredient list—no matter the purpose—calls for a good look at safety, function, and how it affects health over the long haul. Sodium 5-oxo-DL-prolinate comes up from time to time, mostly because it turns up in scientific literature in the context of flavor enhancement or preservation. Still, the idea of putting anything new into what we eat should get people thinking.
This sodium salt, a derivative of pyroglutamic acid, shares some characteristics with other flavor modifiers and amino acid derivatives. It tends to fall in with those compounds used to enrich or round off tastes, especially savory profiles. Some technical reports note its potential as a seasoning ingredient or as a way to tweak mouthfeel and taste depth in processed foods.
Nutritionally, the base compound sits within the human metabolic pathway. Pyroglutamic acid plays a part in the gamma-glutamyl cycle, which links closely to basic biochemical processes we rely on every day. This kind of building block often draws interest for its apparent “naturalness.” Still, natural doesn’t always translate to safe or beneficial at the levels used in food processing. Lab studies look promising in limited aspects, but thorough human testing remains thin on the ground.
Checking regulatory guidelines is the smart move before any additive reaches the shelf. The big food safety agencies in the US and Europe—namely, the FDA and EFSA—keep extensive lists of approved food additives. As of now, sodium 5-oxo-DL-prolinate has not picked up a broad approval for use in foods from either group. Without an official “Generally Recognized As Safe” (GRAS) status, companies face big hurdles in marketing food with this ingredient.
Without ample data from human trials or long-term consumption studies, predicting the risks stays tough. Scientists flag the need for allergenicity testing, toxicological assessments, and an understanding of possible impacts on kidney or liver function, especially in vulnerable populations like children, pregnant people, and those with metabolic disorders. Rash introduction of new additives in the past—notably those that went out of fashion due to health concerns—should keep food makers cautious.
People want to know what’s in their food. Brands gain consumer trust by embracing transparency and clear labeling, especially with new ingredients. Pushing for more independent research before commercial launch keeps public health front and center. If future studies show no adverse effects, food producers interested in novel taste improvements might revisit sodium 5-oxo-DL-prolinate, leaning on the data rather than claims.
Cooking at home, I always check ingredient panels. Any item I can’t pronounce or find well-supported by health authorities raises a flag. Most people do the same, even if they don’t always have time to scrutinize everything. If a new additive can’t point to strong evidence of its safety, it doesn’t belong in my kitchen. Whether it’s for taste, shelf life, or cost, the burden falls on the food industry to prove something is safe beyond doubt. This isn’t just about following rules—it’s about real lives.
Sodium 5-oxo-DL-prolinate has found its way into skincare for good reasons. This amino acid derivative can help skin hold onto water, offering a more hydrated feel after application. But not every formula will benefit from loading up a ton of it. Instead, careful attention to concentration can shape both performance and safety.
Most formulators seem to agree that the sweet spot for sodium 5-oxo-DL-prolinate lands between 0.5% and 3%. Research and product listings from ingredient suppliers reinforce this. It’s not just about maximizing the claim on the label. Go too low, and you don’t get enough of those hydrating perks. Go too high, and it may lead to sticky texture or, in rare cases, mild irritation, especially outside rinse-off products.
European Commission regulations and safety data also back up that 3% mark as an upper limit for most leave-on personal care formulas. You rarely see serious reactions at concentrations used in day-to-day items. Still, patch testing isn’t a bad step, particularly for sensitive skin types.
Not every product needs the same amount. Light facial mists do well with around 0.5% — enough to make a difference without changing feel on skin. Moisturizing creams often work best at 1% to 2%, giving more hydration but not turning sticky or heavy. Some masks or specialized overnight treatments reach toward the higher range, near 3%, but this takes extra care to balance with other ingredients.
Toner formulas stick to the lower end, especially for those targeting quick absorption. Hair care shares some of the same logic. Shampoos and conditioners go up to 3% when a boost of slip and manageability is the focus. Lower levels suit leave-in products where buildup can be a concern.
Studies support using sodium 5-oxo-DL-prolinate in the low percentage range for improving the skin’s ability to retain moisture. According to published cosmetic science literature, effects show up at concentrations as low as 0.2%, but consumer feedback highlights better results above 1%. Producers like Evonik and other ingredient suppliers consistently point to that 0.5%-3% window in their safety and efficacy information.
Dermatologists and cosmetic chemists share their preference for staying under 3% in leave-on items, a view based on patch testing and feedback from real-world use. Most irritation problems have happened only at concentrations well above what’s usually found on retail shelves.
Adding more isn’t always better. Pushing past 3% doesn’t guarantee a softer or more hydrated finish, and may tip the formula over into that gritty or slippery territory that turns customers off. Some brands run into issues by stacking multiple humectants or buffering agents, making the finished product unpleasant. Careful attention to the ingredient deck, and testing on a range of skin types, avoids most of these problems.
Keeping sodium 5-oxo-DL-prolinate at or below 3% helps maintain both comfort and safety. Balancing with other moisture-attractors like glycerin or hyaluronic acid lets you showcase the power of each, rather than overwhelming the skin. Taking time for stability testing pays off here, making sure no unwanted reactions or texture shifts show up in final packaging.
Plenty of innovation still happens with an ingredient as simple as sodium 5-oxo-DL-prolinate, but respect for recommended levels keeps everyone — from chemist to consumer — happy and protected.
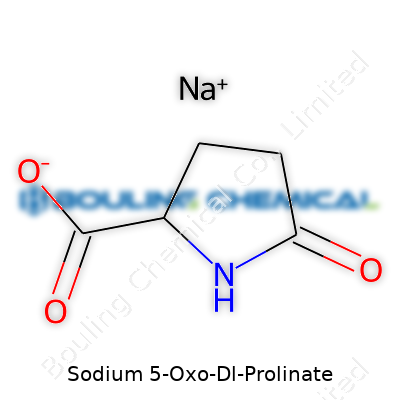
| Names | |
| Preferred IUPAC name | Sodium 5-oxopyrrolidine-2-carboxylate |
| Other names |
DL-Pyroglutamic acid sodium salt DL-Pyroglutamate sodium DL-5-Oxoproline sodium salt DL-Pyrrolidonecarboxylic acid sodium salt |
| Pronunciation | /ˌsoʊdiəm faɪv ˈɒk.soʊ ˌdiː ɛl proʊˈlɪneɪt/ |
| Identifiers | |
| CAS Number | 66192-28-9 |
| 3D model (JSmol) | `3D structure; ready; model; C1CC(=O)NC1=O.[Na+]` |
| Beilstein Reference | 172399 |
| ChEBI | CHEBI:91251 |
| ChEMBL | CHEMBL2104348 |
| ChemSpider | 71313922 |
| DrugBank | DB14596 |
| ECHA InfoCard | 03-211-998-367 |
| EC Number | 2523-06-2 |
| Gmelin Reference | 83409 |
| KEGG | C01879 |
| MeSH | D020123 |
| PubChem CID | 12518504 |
| RTECS number | TC9659600 |
| UNII | X960YZ4F0P |
| UN number | 2811 |
| Properties | |
| Chemical formula | C5H8NNaO3 |
| Molar mass | 157.11 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 1.345 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -3.0 |
| Acidity (pKa) | 1.82 |
| Basicity (pKb) | 7.95 |
| Magnetic susceptibility (χ) | -41.5e-6 cm³/mol |
| Refractive index (nD) | 1.490 |
| Dipole moment | 8.47 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 157.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -778.5 kJ/mol |
| Pharmacology | |
| ATC code | N02BG07 |
| Hazards | |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD50 (oral, rat): 4730 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Sodium 5-Oxo-Dl-Prolinate: "5050 mg/kg (rat, oral) |
| NIOSH | WH7400000 |
| REL (Recommended) | 5 mg/kg |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Proline Pyrrolidone carboxylic acid DL-Pyroglutamic acid Sodium glutamate Glutamic acid |