Chemical curiosity has always pushed scientists to dig into the structures hiding on the fringes of expected chemistry. The story of (S)-Pyrrolidine-2-Carboxamide mirrors the broader saga of amino acid analog discovery. Decades back, folks looked at natural proline and saw potential spin-offs laying groundwork for newer, more specialized molecules. Tinkering in labs, researchers isolated the (S) enantiomer, recognizing its distinct spatial arrangement gave unique biological properties. The rise of asymmetric synthesis and chiral technology in the 90s really gave a jolt to its study, and with the boost in peptide research and pharmaceutical development, this compound picked up attention thanks to its role as a building block, chiral auxiliary, and starting point in the synthesis of complex molecules.
The molecule packs versatility in a fairly simple framework. (S)-Pyrrolidine-2-Carboxamide falls under the amino acid derivatives, dressed up from proline by turning the carboxyl group into a carboxamide. Chemists pick it for reactions that need chiral guidance, pharmacologists eye it thanks to its presence in peptide mimics, and material scientists favor its stability and ability to engage in hydrogen bonding. Many commercial variants appear as off-white to pale yellow solids, with purity standards ranging from research-grade to pharma-grade.
Once you hold a sample, you notice the crystalline or powder form, typically stable under room conditions, with melting points falling somewhere between 150°C and 170°C, depending on crystal hydration or any lingering impurities. The solubility profile makes it suitable for both aqueous and some organic solvents; polar aprotic solvents seem to favor dissolution. The molecule stands up reasonably well to air and moisture, attributes that simplify handling and storage. On the chemical side, the amide linkage can open doors for condensation reactions, while the pyrrolidine ring maintains rigidity and some degree of reactivity on the nitrogen, especially useful for directed synthesis or functionalization.
A proper batch walks in weighing documentation and precise labeling—a testament to quality assurance, not bureaucracy. Purity gets cited in percentage, often HPLC-verified above 98%. Labels specify stereochemistry, which protects against downstream mistakes where the R-isomer or racemic mixture could undermine the goal. Storage advice doesn't meander: keep sealed, away from direct light, and control moisture exposure. Some producers go further, listing heavy metal content, residual solvents, and batch traceability to comply with pharmaceutical and research standards.
My own experience tells me that synthesis tends to start from L-proline, a natural, chirally pure source, converting the carboxyl function to an amide through reagents like carbonyldiimidazole or directly with aqueous ammonia under coupling conditions. Racemic pathways see less use in chiral applications, but they crop up in materials science or bulk chemistry. Purification by recrystallization, chromatographic techniques, or sometimes lyophilization targets excess reactants, tars, and color bodies.
This compound invites experiments with ring modifications, N-alkylations, or introduction of substituents at the 2-position. I’ve seen colleagues develop analogs by oxidation at the pyrrolidine core, tapping the amide as a leaving group in select reactions, or extending the chain through peptide bond formation. (S)-Pyrrolidine-2-Carboxamide also works in cross-coupling with various halides, and serves as a scaffold for β-turn mimics in peptide chemistry. None of these transformations are purely academic—functionalized compounds often become linchpins in target molecule syntheses.
This compound avoids confusion with a handful of aliases: (S)-2-Carbamoylpyrrolidine, L-Pyrrolidine-2-carboxamide, and its IUPAC moniker, (S)-Pyrrolidine-2-carboxamide, among commercial product names like (S)-Proline amide. A glance at catalogs reveals all these labels, proof that even straightforward molecules carry a tangle of trade and scientific identities. CAS numbers, though not printed here, remove ambiguity in paperwork and procurement.
Safety often gets less attention with 'ordinary' molecules, but responsible practice leaves no gaps. Most safety data suggests low acute toxicity, no marked irritation or sensitization on skin contact, and manageable dust risk. Goggle and glove habits never go out of fashion, and dust masks still make sense in bulk settings. Disposal appears less restrictive, following local protocol for non-halogenated, non-volatile organics. Mindfulness grows sharper when working in pharmaceutical pipelines; documentation expands to spill response, cross-contamination, and trace impurity control.
Pharmaceutical research circles keep rediscovering uses for (S)-Pyrrolidine-2-Carboxamide. Its principal calling card sits with peptide synthesis, where chiral purity ensures fidelity in building protein-mimicking chains. Medicinal chemists value the scaffold for tweaking bioactive compounds—sometimes as direct analogs, sometimes as rigidifiers to probe SAR relationships. Beyond peptides, enzyme inhibitors and CNS-targeting agents draw on the proline core’s properties. Analytical labs use the molecule to check chiral separation techniques. Industrial settings stretch its reach to specialty polymers and certain agricultural actives, where its backbone delivers both structure and function.
The last ten years spun new research angles. Academic projects illustrate the compound’s adaptability as part of combinatorial libraries, diving deep in discovery of enzyme interactors or signaling pathway probes. Advances in computational chemistry throw light on its binding tendencies, predicting outcomes before reagents even hit the flask. Several research groups deploy (S)-Pyrrolidine-2-Carboxamide in photochemical studies, exploring how altered electronic structures shape new dyes and molecular switches. Many patents cite it as an intermediate in key syntheses for pipeline molecules.
There’s that question that lands on every lab desk—how safe is it really? Historical records didn’t outline substantial acute toxicity in mammalian models, but chronic exposure data remains patchy. Cell studies noted minimal cytotoxicity at the concentrations typical in peptide synthesis. Still, rare impurities or overreliance on structural analogies shouldn’t lull anyone. Proper record-keeping and fresh periodic reviews help prevent surprises as new applications emerge. The compound’s amide group suggests relatively low metabolic reactivity, but ongoing metabolism studies fill the gaps. Regulatory attention feels poised to step up if new uses bring exposure to sensitive populations.
Demand shows every sign of rising. The surge of peptidomimetic drug types—think new antivirals or targeted oncology drugs—depends on solid, affordable chirality sources. AI-driven drug design searches out modified proline derivatives, and (S)-Pyrrolidine-2-Carboxamide joins hit lists for its blend of rigidity and functional adaptability. Advances in green chemistry push for even cleaner synthesis from renewable proline or biocatalytic routes. Down the road, improvements in resolution technology and more rigorous safety profiling set the stage for better, safer applications across biotech, pharma, and even material science. As the molecule shifts from fine chemical oddity to essential toolkit staple, open research culture and data sharing will become the main levers for next breakthroughs—ones that could push the humble amide ring into new therapeutic or industrial territory.
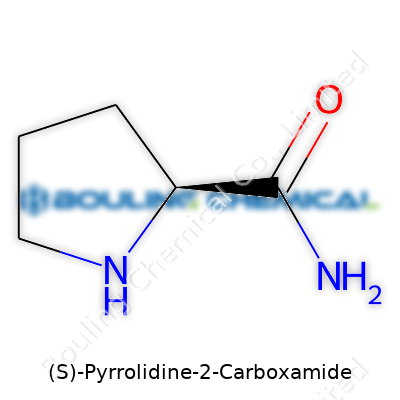
(S)-Pyrrolidine-2-Carboxamide, a mouthful to say, often pops up in scientific circles, yet regular folks seldom hear about it. This compound grabs attention because it plays a part in the world of drug and chemical research. Its structure—a pyrrolidine ring with a carboxamide group—sets it apart as a handy building block for making more complex molecules.
I’ve seen researchers use (S)-Pyrrolidine-2-Carboxamide as a stepping stone in the lab. Drug discovery teams rely on building blocks like this one when piecing together molecules that target diseases. It brings chiral (one-handed) features to molecules, which turns out to be crucial. Many drugs succeed or fail based on their “handedness.” For example, some HIV antiretroviral drugs were only effective because their molecules had the right twist. Adding a group like (S)-Pyrrolidine-2-Carboxamide lets chemists target that kind of precision early in the design process. Getting chirality right can mean the difference between a medicine helping or hurting.
Lab experience showed me that not all chemicals work out-of-the-box. Researchers wage a daily battle for purity and right-handedness—two things that (S)-Pyrrolidine-2-Carboxamide delivers. A misstep with the wrong form of an intermediate can stall the development of a new drug, costing time, money, and energy. This compound spares teams from those wasted efforts. It’s rare to see headlines about a reagent or intermediate, yet so many medical breakthroughs depend on these behind-the-scenes building blocks. Even minor tweaks to an intermediate like this can change the properties of the finished compound, shifting how the body absorbs or breaks down a prospective medicine.
(S)-Pyrrolidine-2-Carboxamide doesn’t appear on pharmacy shelves. Instead, it gets stitched into much larger molecules. For example, chemists can use it to build peptides or small-molecule pharmaceuticals, especially those aimed at enzymes or receptors sensitive to the shape of a molecule. Think about blood pressure medications, anti-seizure drugs, or experimental cancer treatments; many started off as sets of tiny hand-picked building blocks brought together in the right sequence. This approach stops unwanted side effects by ensuring drugs interact with their targets and not with other cells.
Producing this compound at scale has never been simple. Labs must juggle the demand for chirality, high purity, and consistency. In my time working with pharmaceutical chemists, I learned about the headaches caused by inconsistencies between batches. A single contaminant—or a small amount of the wrong hand—can throw a whole project off track. Suppliers have started to improve their purification and testing methods, drawing from high-performance chromatography and advanced spectroscopy. Researchers insist on detailed certificates of analysis and regular audits of production plants. Working closely with trusted suppliers, sharing testing data, and enforcing tight quality controls have already improved reliability and safety in labs that use these intermediates.
Demand for more precise drugs continues to grow. Research teams lean on building blocks like (S)-Pyrrolidine-2-Carboxamide to create new therapies that work better for patients and create fewer side effects. By putting the right chemistry at the foundations, chemists can build stronger and more trustworthy medicines—ones that make a difference in real lives.
Ask any chemist about pyrrolidine derivatives, and they’ll tell you the basics right off the bat. For (S)-Pyrrolidine-2-Carboxamide, the chemical formula is C5H10N2O, and the molecular weight clocks in at 114.15 g/mol. That small number hides a world of chemical context and utility. Knowing these numbers means more than filling out a label or ticking a regulatory box; you get a concrete sense of what you’re working with—down to the last atom.
I've spent time hunched over a lab bench, pipetting out solutions and watching crystals form. Working with small molecules like this carboxamide highlights how chemistry ripples into daily experience. The molecular weight tells you how much to weigh out for a reaction. The formula reveals details—five carbons, a tight pyrrolidine ring, two nitrogens hinting at amide chemistry. That ring changes everything about how (S)-Pyrrolidine-2-Carboxamide behaves; it's not some open-chain amide, it’s caged, physically and chemically. That’s why it stands out in synthesis.
In the last decade, labs and companies have grown more aware of chirality. The (S) prefix isn’t ornamentation—it's a badge. The “S” version interacts differently with enzymes and receptors. That precise mirror-image structure shapes drug safety and function. The thalidomide scandal still echoes through the halls of pharmaceutical R&D. Regulators and research teams have learned through harsh lessons that one enantiomer can do the job, while the “wrong” hand can cause harm. Responsible chemists need to know exactly which isomer they’re holding before pushing forward.
Research points to the value of these small molecules—(S)-Pyrrolidine-2-Carboxamide isn’t just a brick in drug discovery, it’s a cornerstone for building peptidomimetics, catalysts, and specialty materials. In 2021, market reports showed continued demand for chiral building blocks, citing how they enable novel antivirals and CNS-active agents. Getting these molecules ready, pure, and properly characterized matters for quality, safety, and cost.
Every chemist faces hurdles with availability and reproducibility. Purifying and verifying the specific (S)-enantiomer calls for skill, patience, and tools—polarimeters, NMR, chiral columns. Buying certified reagents, investing in analytical equipment, and developing careful documentation routines help overcome mix-ups or contamination. Training fresh hands to respect these details makes all the difference.
Waste management and sustainability deserve a spotlight. Making chiral molecules often means extra steps and solvents. Some labs in my network switched to greener ways: enzyme-based catalysis instead of relying on metal-heavy reagents, minimizing petroleum-based chemicals. That shift isn’t just good for the planet; it usually cuts costs and headaches.
Years of lab work taught me never to ignore the humble chemical formula or dismiss the detail tucked behind a molecular weight. Care with these fundamentals means progress for research, manufacturing, and even medicine. Tackle the basics, understand the molecule, and chemical insight will follow.
Walking into a lab or thumbing through a catalog, you start to notice there’s usually more than one grade for any compound worth talking about. (S)-Pyrrolidine-2-Carboxamide is no exception. Finding it in different purity grades isn’t just some technical detail. It lines up with real demands in both research and manufacturing.
Work in pharma and your shoulders carry the load of stringent standards. Drug development is personal, affecting people’s health. Nobody wants the risk of a contaminant showing up. So, research teams often hunt for something called “high-purity” or “analytical grade” (S)-Pyrrolidine-2-Carboxamide. Here, purity levels stretch above 98%. I remember a friend working in pre-clinical development telling me that anything less could throw the whole project off course. Impurities might not yell at you from the get-go, but they muddy results, introduce side reactions, and turn regulatory paperwork into a nightmare.
Not every project calls for the highest standard. In basic chemical synthesis or teaching labs, folks don’t shell out for pharmaceutical grade. Lab-grade tends to hover in the 95–98% range. Yes, that little sliver of impurity might make a difference if you’re developing a medicine, but in a classroom or for broad exploratory experiments, the cost savings matter. I’ve seen budgets stretched thin, and most undergrad labs use these less pure samples just to keep the lights on. This grade still lets students and hobbyist researchers get practical exposure to synthesis and analysis.
Strict oversight pushes companies toward higher grades in certain industries. Pharmaceuticals, as I saw with a small generics manufacturer, usually means full documentation and certificates of analysis. A batch of 99% pure compound with all the little impurity peaks accounted for helps labs stay in line with agencies like the FDA or EMA. The stakes ride high: legal consequences, supply chain delays, or worse, patient risk signal that cutting corners on purity can’t happen.
In my own experience running assays, purity was more than a number. Lower-grade samples brought more troubleshooting. Chromatograms grew messy, interpretation slowed down, and sometimes it led to ordering new material and starting over. Multiple colleagues across research disciplines have told me the same story: pay for higher purity up front or risk losing much more in lost time and wasted reagents.
Suppliers hardly keep it a secret that purity grades vary. Honest suppliers publish comprehensive datasheets, often showing HPLC traces and impurity profiles. If in doubt, pick up the phone and ask for documentation. Custom synthesis houses sometimes offer custom purifications. Comparing suppliers helps; not all “98% pure” labeled compounds measure up equally when scrutinized with sensitive instruments.
As online marketplaces grow, there’s more transparency—and more opportunity for confusion. Reliable vendors invest in strong quality systems. Asking about certificates, batch traceability, and previous customer experiences gives insight. In settings where every decimal point matters, verification by an in-house analytical team can catch surprises before they become setbacks.
End of the day, choosing the right purity for (S)-Pyrrolidine-2-Carboxamide isn’t a throwaway decision. The context—be it discovery chemistry, industrial production, or drug development—shapes what counts as “pure enough.” Every project, and every researcher, faces that calculation between risk, rigor, and resource.
Anyone who has worked in a chemistry lab knows how a tiny slip in storage protocol can mess up months of hard work. (S)-Pyrrolidine-2-carboxamide, a compound that often crops up in synthesis projects and enantioselective studies, stands as a classic example of a sensitive material requiring real attention. If you give this compound the wrong conditions, results can go sideways and, worse, you might end up with costly replacements or safety concerns.
Researchers and handlers talk a lot about stability and shelf life, but experience says those are just the surface details. (S)-Pyrrolidine-2-carboxamide tends to attract water from the air. Leave it uncapped or toss it on a warm shelf, and the quality drops fast. Moisture grabs hold and begins to degrade purity. I've seen cases where a poorly sealed container left on a benchtop took on moisture in a few days, which meant the compound no longer performed in reactions as expected. This kind of thing can really set back entire projects.
For (S)-Pyrrolidine-2-carboxamide, dry and cool conditions work best. Most lab guides and chemical suppliers highlight temperatures between 2°C and 8°C. A dedicated refrigerator keeps fluctuations much smaller, which matters because every temperature swing risks condensation and chemical breakdown. Common sense applies: don’t stack it beside reagents that release moisture, and resist the urge to store it in high-traffic compartments where the door gets opened all day.
Use airtight containers without hesitation. Amber vials make sense, since exposure to light—especially in glass-clear packaging—can kick off slow changes in many organic compounds. Simple amber glass with a tight Teflon-lined cap keeps humidity and light away, plus it offers physical protection against accidental spills or wipes. If you can, use a desiccant pack inside the storage box. Silica gel or molecular sieves pull any stray water that sneaks in when the container gets opened.
Rushing, forgetting to re-cap, or tossing containers into the wrong temperature zones ruins more samples than most realize. Sometimes assistants grab the nearest empty vial without thinking about light protection. Years of lab routines have shown that small habits—labeling clearly, using secondary containment, documenting storage dates—help avoid surprises. More than once, a busy researcher or student assumed everything in the fridge would be fine, only to discover cross-contamination or solvent loss from improper closure. It pays off to check seals and rotate older stock so nothing gets stale before use.
Beyond quality, correct storage ties directly to safety. If the compound breaks down or absorbs water, the by-products or altered chemical properties could introduce unknown hazards. Material Safety Data Sheets (MSDS) detail risks, but nothing replaces critical hands-on habits. Regularly review storage practices and double-check temperature logs, especially during power outages or equipment failures. It’s not just about preserving chemistry—a safe storage routine backs up compliance with institutional policies, keeps audit trails clean, and supports the reproducibility that good science demands.
Real-world experience shows a little extra care with (S)-Pyrrolidine-2-carboxamide storage pays off in reliable reactions, less waste, and fewer headaches for the whole team. Good protocols start with fundamentals and don’t cut corners on details as simple as a tightly closed lid or a clean, cool shelf.
Chemistry doesn’t have room for shortcuts, especially with substances like (S)-Pyrrolidine-2-Carboxamide. In research labs, this compound helps create advanced pharmaceuticals and specialty chemicals. Yet, safety often gets brushed aside once the novelty fades. That’s when accidents sneak up. Having handled similar cyclic compounds myself, I've seen small spills get out of hand because folks didn't respect the basics.
The smell of a fume hood and the snap of latex gloves signal the start of a safe experiment. No matter how familiar the procedure feels, gloves, goggles, and a crisp lab coat each play a role in keeping chemicals away from skin and eyes. I learned this the day a minor solvent splash led to a nasty irritation, which could have been worse with more reactive chemicals like (S)-Pyrrolidine-2-Carboxamide.
Even if a datasheet lists it as a mild irritant or “not acutely toxic,” don’t lower your guard. Fine dust lingers and powder clouds often escape notice until a cough signals exposure. Respirators or dust masks help, especially in poorly ventilated spaces or during weighing and transfer. Tight, well-sealed containers keep the powder from escaping, which helps keep the air clear and safe.
My mentor drilled this into me: put things back after use and label them right. (S)-Pyrrolidine-2-Carboxamide should stay in a cool, dry spot, away from moisture and light. Any sign of clumping or color change signals contamination or decomposition—don’t risk it, toss it properly. I’ve seen more than one project ruined because someone left a reagent cap loose for a morning coffee break.
Keep incompatible chemicals apart. Strong acids, bases, and oxidizers have no business mixing with your stock of (S)-Pyrrolidine-2-Carboxamide. Mixing can trigger exothermic reactions or release unknown byproducts, threatening people and research alike.
No one forgets the first chemical spill. The sound of powder hitting the bench is a stomach-drop moment. For spills, sweep up solids gently—avoid stirring up dust—and use damp paper towels for residues. Always put cleanup waste in a labeled, sealed bag or bottle, then send it out with regular chemical waste. Sink disposal is off-limits; municipal water systems can’t process synthetic compounds safely.
Before your first batch, set up emergency steps with your team. Know the eyewash, keep MSDS sheets close, and review fire procedures. I‘ve seen teams stall in confusion because nobody reviewed these simple steps for months at a stretch.
There’s nothing soft about asking questions or revisiting protocols. Even seasoned researchers need refreshers, especially with less common compounds like (S)-Pyrrolidine-2-Carboxamide. Monthly reviews help settle disputes and close knowledge gaps. If someone doesn’t know what to do, that’s not a weakness—it’s the start of better safety. I’ve seen open discussion save hundreds of hours and more than a few injuries.
Every lab culture builds itself around daily habits. Gloves get worn, lids get closed, and old chemicals head for the waste bin, not the forgotten shelf. That discipline protects people and projects. Safe handling is common sense, but common sense isn’t common unless you practice it every single day.
| Names | |
| Preferred IUPAC name | (S)-Pyrrolidine-2-carboxamide |
| Other names |
(S)-2-Pyrrolidinecarboxamide L-Pyrrolidine-2-carboxamide |
| Pronunciation | /ˈɛs paɪˈrɒlɪdiːn tuː kɑːrˈbɒksəmaɪd/ |
| Identifiers | |
| CAS Number | 74911-35-8 |
| 3D model (JSmol) | `3D model (JSmol) string` for **(S)-Pyrrolidine-2-Carboxamide**: ``` CC1CNC(C1)C(=O)N ``` |
| Beilstein Reference | 120715 |
| ChEBI | CHEBI:15611 |
| ChEMBL | CHEMBL138865 |
| ChemSpider | 5464418 |
| DrugBank | DB03044 |
| ECHA InfoCard | 31e3fd6a-de2e-4def-b8e8-2c3dfe470266 |
| EC Number | 68290-83-5 |
| Gmelin Reference | 107179 |
| KEGG | C11410 |
| MeSH | D017210 |
| PubChem CID | 12017379 |
| RTECS number | UY9655000 |
| UNII | V5A27P4S7A |
| UN number | 2811 |
| CompTox Dashboard (EPA) | DSSTox_CID_42801004 |
| Properties | |
| Chemical formula | C5H10N2O |
| Molar mass | 114.15 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.14 g/cm3 |
| Solubility in water | soluble |
| log P | -2.0 |
| Vapor pressure | 0.000203 mmHg at 25°C |
| Acidity (pKa) | 11.27 |
| Basicity (pKb) | 8.97 |
| Magnetic susceptibility (χ) | -68.3 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.496 |
| Dipole moment | 4.01 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 89.6 J·mol⁻¹·K⁻¹ |
| Hazards | |
| Main hazards | Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05, GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 104.5 °C |
| Lethal dose or concentration | LD50 (oral, rat) > 5000 mg/kg |
| NIOSH | NJ4A28661U |
| PEL (Permissible) | No PEL established. |
| REL (Recommended) | 10 mg |
| Related compounds | |
| Related compounds |
Pyrrolidine Pyrrolidine-2-carboxylic acid L-Prolinamide (R)-Pyrrolidine-2-carboxamide Proline Pyrrolidinecarboxamide |