Chemists have pushed the boundaries of organic synthesis for decades, searching for intermediates to build everything from advanced drugs to specialty chemicals. (R)-(+)-N-Boc-3-Pyrrolidinol can trace its roots back to the surge in chiral auxiliary design in the late 20th century, a period when separating enantiomers became crucial for pharmaceutical research. This molecule emerged not from a burst of fame, but from a steady rise in asymmetric synthesis strategies. Early patents and published routes pointed to clever use of enantioselective resolution, which proved instrumental once the pharmaceutical sector ramped up demand for single-enantiomer compounds. Its journey reflects the broader trends in using protecting groups for selective reactions and the constant push for improved yields in chiral building blocks.
Take a close look at (R)-(+)-N-Boc-3-Pyrrolidinol and its structure tells a clear story. The Boc group shields the nitrogen, keeping unwanted side reactions in check during synthesis. This makes it far easier for researchers to access otherwise touchy intermediates. It acts as a key stepping-stone in making more complex nitrogen heterocycles. Labs worldwide acquire this compound not as a showpiece but for its reliability and versatility in drug design, crop protection, and other specialty material domains. Its value stems directly from the way it streamlines process development, saving weeks or months that might have been lost to purification headaches.
You’ll find (R)-(+)-N-Boc-3-Pyrrolidinol as a colorless or slightly off-white crystalline solid, usually carrying a faint, amine-like odor. It melts in the moderate range, floating around 50–60°C, and dissolves well in most polar organic solvents—think dichloromethane, acetonitrile, or THF. Its chiral center enjoys solid stability under standard storage, thanks to the Boc group dampening degradation pathways. In solution, it keeps its integrity both under mild acidic and basic conditions for practical bench handling. Anyone working with this intermediate can rely on standard lab techniques for handling, but moisture and acids should stay away from prolonged contact to avoid deprotection.
As far as the technical sheet reads, purity sits above 98%, often verified by chiral HPLC and NMR. Reputable suppliers test both optical rotation and IR to show absence of major contaminants or racemization. Labels usually mark the CAS number, batch reference, date of synthesis, and key safety information like irritant risk, as per GHS standards. Shipping containers protect the sample from light and damp, but standard amber glass vials suffice for lab stocks. Reliable documentation keeps quality assurance straightforward for anyone tracing lots back to origin.
Most lab-scale syntheses begin with commercially available pyrrolidinone, which first gets Boc-protected on nitrogen, typically using di-tert-butyl dicarbonate and a base, such as triethylamine. Reduction of the lactam opens the ring to the corresponding alcohol, carried out with the likes of borane–THF or selective catalytic hydrogenation. The last step involves chiral resolution, often with chromatography or enzymatic methods, to isolate the (R)-enantiomer. Process development groups focus attention on atom economy and route optimization, cutting down on waste whenever possible and investing in chirally pure raw materials to increase efficiency.
Once in hand, (R)-(+)-N-Boc-3-Pyrrolidinol handles further derivatization with confidence. The free alcohol function serves as a handle for etherification, acylation, and oxidation. Boc removal under mildly acidic conditions liberates the pyrrolidine, which can be slotted into peptide synthesis or other amide-forming steps. Skilled chemists leverage this dual functionality to create a broad range of derivatives, especially where both chirality and functional group tolerance matter. Pharmaceutical labs have made use of this flexibility in building blocks for CNS-active drug candidates, among others.
The chemical catalogues and research papers reference this compound under aliases like (R)-3-Hydroxy-N-Boc-pyrrolidine, (R)-3-Pyrrolidinol, N-Boc protected, or tert-butyl (3R)-3-hydroxypyrrolidine-1-carboxylate. Different vendors may toss in their unique identifiers, but the structure and chiral descriptor—(R)—remain non-negotiable for clarity and ordering accuracy.
Any researcher working with (R)-(+)-N-Boc-3-Pyrrolidinol should wear gloves, goggles, and a lab coat. Dust can irritate skin and eyes, so fume hoods see regular use during weighing or transfers. Material safety data sheets flag the main concerns: skin or respiratory tract irritation and the low risk of fire from organic dust. Standard waste collection goes through the usual organic solvent bins, but no additional hazard flags set it apart from typical amine/alcohol combinational intermediates.
Both academia and industry use this compound as a backbone for synthesizing chiral alkaloid analogs, beta-lactam antibiotics, and certain anti-viral agents. Agrochemical innovation groups use it to piece together new classes of crop protectants or growth regulators. Each year, medicinal chemistry teams turn to pyrrolidine derivatives like this one as building blocks or fragments to set chiral centers early in drug leads. Its protected architecture speeds up route scouting and late-stage functionalization.
In the search for new pharmaceuticals, chiral intermediates drive early project momentum. Researchers prefer molecules like (R)-(+)-N-Boc-3-Pyrrolidinol due to the straightforward way it connects to diverse compounds—azetidines, beta-lactams, and peptidomimetics emerge out of its functional handles. Advances in green chemistry have sparked innovation in its preparation, with biocatalysts and flow synthesis streamlining the route. Process chemists monitor and tweak these steps to cut down on byproducts and lower overall costs, looking to meet both regulatory and scale-up needs without throttling throughput.
Animal studies and in vitro work have largely found this molecule to pass toxicity screens at standard lab concentrations. The Boc group dampens reactivity, so cellular assays don’t show mutagenicity or acute toxicity under routine exposures. Regulatory filings reflect this, with hazards linked more to general amine and alcohol properties—irritation risk, not cytotoxicity or long-term organ damage. Larger data sets still remain limited, but downstream metabolites of the parent pyrrolidinol structure haven’t flagged any warning signs in the most recent screenings.
Growing demand for enantioselective syntheses guarantees a place for (R)-(+)-N-Boc-3-Pyrrolidinol in discovery chemistry. Synthetic efficiency continues to improve, especially with automated peptide synthesis and green processes lowering cost per gram. Expanding fields, including advanced materials and biological probes, see this chiral intermediate offering fresh opportunities for rapid prototyping and molecular design. Investments in sustainable chemistry pave the way for more environmentally gentle routes, which will make large-scale production more feasible without trade-offs in purity or safety. The pipeline for next-generation medicines, particularly targeting neurological diseases and viruses, will keep this compound and its derivatives in active play, as researchers adapt to overcome resistance trends and push drug candidates forward.
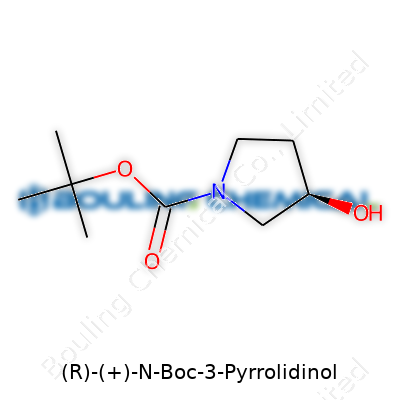
Ask anyone who spends their days at the bench: chemical names like (R)-(+)-N-Boc-3-Pyrrolidinol look complicated, but each piece means something to the person mixing, distilling, or crystallizing in a lab. Here, you find a molecule rooted in the five-ringed structure called pyrrolidine, with an alcohol group tagging along and a bulky Boc (tert-butoxycarbonyl) group parked on the nitrogen.
The real twist lies in its handedness — that R-(+) prefix flags a specific spatial arrangement known as a stereoisomer. Chemists use this detail every day because living systems treat right- and left-handed molecules as different. My own early days in the lab taught me that, in practice, chirality doesn’t just influence basic research. One version of a molecule can fit into an enzyme like a key, while its mirror image might slide by without effect or even trigger entirely different responses.
Strip the fancy name back: you’ve got a pyrrolidine ring, with the number three carbon carrying an alcohol (–OH) group. The nitrogen wears the Boc protecting group, a trick used by chemists to keep sensitive nitrogen atoms from reacting before their time. The Boc group comes off easily with acid, like peeling away tape once the job’s done.
Structurally, the molecule’s backbone looks like this: a five-membered ring of four carbons and one nitrogen atom. Attach the Boc group to the nitrogen, then place the alcohol on the third carbon, not the first or the fourth — chemistry works with exactness, not guesswork. Where each group sits defines what the molecule can do, whether in drug synthesis or materials design.
For those in organic synthesis, picking the right enantiomer (R or S) saves effort later, especially if you're building something complicated like a pharmaceutical. Companies pour resources into finding the most efficient, reliable routes to these chiral building blocks. In the case of (R)-(+)-N-Boc-3-Pyrrolidinol, every milligram counts because downstream steps rely on having the R-enantiomer and not its opposite.
That specificity keeps side products in check and improves yields, which means safer medicine and fewer headaches for those in scale-up or regulatory work. Research shows that the wrong enantiomer has led to ineffective or even dangerous drugs in the past. Chemistry learned a tough lesson from history and now puts chiral control front and center.
Synthesizing enantiomerically pure compounds doesn’t come easy. Traditional methods can involve inefficient resolution techniques or expensive chiral catalysts. Some chemists have shifted toward using enzymes tailored for specific transformations — these biological catalysts speed things up, cut back on nasty waste, and sometimes work in water, which is a rare bonus.
As demand for chiral intermediates grows, especially in pharmaceuticals and advanced materials, investment in green chemistry and sustainable chiral auxiliaries makes sense. I’ve seen labs tweak conditions and recycle reagents to cut costs and improve safety. The next step could see more open-source sharing of robust synthetic pathways and smarter process monitoring, pushing quality and accessibility forward for researchers everywhere.
(R)-(+)-N-Boc-3-Pyrrolidinol stands as more than a string of atoms. Each protecting group, each specific stereochemistry, shapes its use in the lab. Get the details right, and you open up new possibilities in drug discovery, catalysis, and beyond. Ignore them, and you invite mistakes that waste time and resources, or worse. In chemistry, every small decision ends up mattering, and this compound’s structure proves that truth day after day.
Step into any modern pharmaceutical lab and you’ll spot shelves lined with bottles labeled in fine print — (R)-(+)-N-Boc-3-Pyrrolidinol is one of those specialty ingredients that researchers lean on during complex projects. With its chiral center and protective Boc group, the molecule gives synthetic chemists a flexible starting point while trying to build compounds that demand both selectivity and stability.
In organic chemistry, efficiency means everything. A key reason this compound draws regular use comes from its stable Boc group (tert-butoxycarbonyl), which shields the nitrogen atom. By keeping the nitrogen protected, chemists avoid tricky side reactions during multi-step syntheses, particularly in routes creating chiral pharmaceuticals. From my own bench work, using a Boc-protected intermediate can save hours of troubleshooting later on, especially during scale-up for pilot batches.
The pharmaceutical world lives and dies on the ability to create single-enantiomer drugs. (R)-(+)-N-Boc-3-Pyrrolidinol allows teams to construct these molecules with a high degree of control. You’ll find that this building block pops up in medicinal chemistry projects aiming to make new central nervous system drugs or antiviral compounds, where the “handedness” of the molecule can mean the difference between therapeutic effect and inactivity.
As of 2024, scientists still draw on the lessons from the late 90s and early 2000s, when improved access to chiral intermediates sped up the race to develop new treatments for infections, cancer, and neurodegenerative conditions. Big pharma invests millions in optimizing their synthetic routes, and a reliable intermediate like this makes life easier by underpinning patents for new blockbuster drugs. It helps companies chase regulatory approval with fewer headaches over impurities or unwanted byproducts, giving patients safer and more effective medications.
Drug research often turns to natural products or peptide analogues for inspiration. In these areas, protecting groups serve as a chemist’s toolkit. (R)-(+)-N-Boc-3-Pyrrolidinol, with its predictable reactivity, enters the mix during peptide synthesis, especially in routes that need amino alcohols. In labs specializing in total synthesis — where replicating natural molecules is the challenge — an intermediate like this enables selective modification, helping teams add or remove groups at just the right step. Years ago, while running peptide couplings for a biotech startup, having pre-protected amino alcohols simplified the route and improved our yields. Fewer purification steps meant faster progress toward bioactive prototypes.
Custom chemistry grows more important as personalized medicine gains attention. (R)-(+)-N-Boc-3-Pyrrolidinol feeds that demand by opening up new ways to design and build molecules tailored for specific molecular targets. Whether it’s tweaking a functional group to boost the bioavailability of a lead drug, or generating small libraries of related compounds for screening, this intermediate plays a supporting role in innovation. Its stereochemistry allows for quick generation of analogs — a process central to modern drug pipeline developments.
Trust in any new compound rests on proving it meets strict purity and safety standards. Reagents like (R)-(+)-N-Boc-3-Pyrrolidinol pass through rigorous tests to meet those requirements. Suppliers track impurity profiles, batch reproducibility, and provide analytical reports so that pharmaceutical companies and research organizations feel confident incorporating the building block into regulated work. Experience has taught the value of traceable quality, especially with the risk of failed batches or regulatory setbacks looming in high-stakes environments.
As drug development pushes toward more specialized therapeutics, the role of adaptable intermediates only grows. (R)-(+)-N-Boc-3-Pyrrolidinol stands out where reliability and versatility matter, giving chemists the control needed for success in competitive research. Reliable routes, simplified workflows, and reproducible outcomes will keep this molecule relevant wherever synthetic chemistry tackles health challenges that defy easy answers.
Purity matters a lot. Whether the compound ends up in a pharmaceutical lab or at an industrial scale facility, small impurities often lead to costly failures down the line. For (R)-(+)-N-Boc-3-Pyrrolidinol, the best suppliers deliver material with purity upwards of 98%. Some producers boast GC or HPLC data ticking past 99%. Anything lower, and skepticism starts to creep in, especially for those using the compound as a chiral building block. Synthetic chemists know—even trace contaminants can sideline a project, so checking the certificate of analysis is never a wasted step.
Every batch should come with a specification sheet, and real-world buyers never skip reading it. A typical spec for (R)-(+)-N-Boc-3-Pyrrolidinol includes:
Research and industry sometimes veer in different directions, yet they both depend heavily on reliable quality. I’ve watched projects grind to a halt because a new batch of material, labeled “98% pure,” still failed a QA test. That kind of event doesn’t just waste money. It erodes trust between teams and blows up delivery schedules. Consistent, detailed specification sheets are the only reason that risk stays manageable.
Real verification goes beyond trusting what’s typed on a datasheet. In my own lab years, crude shortcuts like melting point checks caught more out-of-spec batches than I’d like to admit. GC and HPLC trace impurities in a way you just can’t spot by eye or by smell. Analytical chemists become natural skeptics—if a peak looks off, they start troubleshooting before anyone else even notices there’s a problem.
Buying (R)-(+)-N-Boc-3-Pyrrolidinol isn’t just a click-and-receive affair; it works much better with a relationship between buyer and supplier. Communicating up front about analytical requirements and certificates of analysis keeps both sides honest. Responsible suppliers field questions on request. Real service means sending chromatograms, test methods, and batch histories without needing to get management involved.
If purity or any other spec drifts even a bit, users should have confidence that feedback gets action, not just an apology. Improving this transparency builds the kind of trust that keeps time-to-market down and innovation moving forward. As regulations tighten and customers get smarter, only suppliers with solid, evidence-backed specs stick around. The rest fade out, and that’s not an accident.
Lab teams can prevent headaches by running identity and purity checks before large-batch purchases. Investing in reliable instrumentation, partnering with trusted vendors, and insisting on full specification disclosure keeps both costs and stress levels down. If users run into ambiguity, pushing for open dialogue or third-party testing speeds up problem solving.
Every research breakthrough, scale-up, or launch rides on the quality of foundational materials. Consistent, carefully specified (R)-(+)-N-Boc-3-Pyrrolidinol isn’t just a chemist’s wish list item—it’s the lifeblood of a project built to last.
Anyone who's ever handled (R)-(+)-N-Boc-3-Pyrrolidinol knows it’s not a pantry item for the faint-hearted. This compound serves both academic chemists and pharmaceutical scientists. With a structure sensitive to a range of conditions, a bit of hands-on care makes a big difference for results and safety. Think of it more like a delicate ingredient in a working lab, not something you can stow away just anywhere.
No one wants to lose an expensive bottle of protected pyrrolidinol to poor storage. If you check the manufacturer’s guidance or scientific supply catalogs, low temperatures stand out. Room temperature causes the compound to break down faster. My own routine means placing it right in a cold spot: a refrigerator ranging from 2 to 8°C keeps the structure intact much longer. Some labs take it a step further and use a -20°C freezer, especially for stock solutions or long-term use. That extra chill helps prevent moisture in the air from nudging the molecule toward unwanted reactions.
After a few mishaps with degraded samples, I picked up one rule: avoid opening and closing the container more than you have to. Direct contact with humid air brings trouble, since (R)-(+)-N-Boc-3-Pyrrolidinol will absorb moisture and eventually degrade. Once water sneaks in, the compound can hydrolyze, breaking the delicate Boc-protecting group. In my own work, I make sure the bottle gets sealed right away after dispensing, using either a screw-cap vial with a gasket or a freshly-wrapped septum. For folks in humid climates, consider dry-box storage or slipping a fresh silica gel packet into the storage container.
This compound doesn’t take well to bright fluorescent lab lighting or direct sunlight, either. Photodegradation is a risk. I toss a piece of foil around the bottle in transit or during longer-term storage. Amber bottles or opaque vials work even better for keeping light away.
Many labs store acids, bases, and oxidizers nearby, but I always keep (R)-(+)-N-Boc-3-Pyrrolidinol separate from them. It reacts with strong acids or bases—common laboratory hazards—to lose the protective Boc group or suffer ring-openings. A shelf just for neutral, non-reactive organics helps avoid accidental spills or fumes spoiling the contents. Label the bottle clearly, and make sure others know its location and storage routine. If you tend to decant sample portions, new vials should be dry, clean, and clearly dated to keep track of shelf life.
The importance of storing this compound right goes beyond dollars and cents. Working with stable chemicals matters for reproducible science and for lab safety. Spilled or degraded (R)-(+)-N-Boc-3-Pyrrolidinol can give off unpleasant fumes or even trigger allergic reactions in rare cases. Using gloves and goggles isn’t just a guideline in the safety manual—after a skin irritation or two, that lesson sticks. Laboratories following good chemical hygiene, using fume hoods for weighing and opening, have fewer accidents and fewer ruined batches.
Getting into the habit of storing sensitive intermediates correctly makes the entire lab more reliable and professional. Keeping a log of purchase date and first use lets you spot anything past its prime. Some labs add periodic quality checks on stock solutions, using thin layer chromatography or NMR, to catch decomposition early. Trust in your samples starts with making storage a careful, routine task.
Buyers in chemical synthesis, pharmaceutical research, and advanced materials keep a steady eye on the supply of unusual chiral reagents. (R)-(+)-N-Boc-3-Pyrrolidinol catches particular interest due to its key role as a stereoselective building block. Challenges tend to surface in the hunt for bulk amounts—not just in price, but in actual procurement. Labs run up against varied lead times, inconsistent purity grades, and minimum order tonnages that don’t always match research or production calendars.
Markets rarely treat one specialty intermediate like another. For substances such as (R)-(+)-N-Boc-3-Pyrrolidinol, availability often traces back to demand cycles in pharmaceuticals or custom synthesis projects. Most standard catalogs list 1g or 5g vials for lab-scale use, but large-scale orders pull supply chains in a different direction. Several European and Asian suppliers list bulk capacities, but buyers should not assume a few online catalog lines equal genuine stock.
In practice, the bottleneck often appears at the commercial synthesis stage. Producing this compound with high enantiomeric purity takes specialized technology—resolution, asymmetric catalysis, or enzymatic transformation. Raw cost rises, and the risk of contamination or batch variance jumps. Only established manufacturers with GMP or ISO-certified plants consistently manage kilogram to ton-scale output without supply hiccups.
Procurement teams face more than price or availability: pharmaceutical-grade chemicals demand reliable stereochemical consistency. Documentation, batch records, and certificates of analysis must line up with project requirements. Without proper vetting, buying bulk can lead to mismatched purity, traces of unwanted isomers, or compliance headaches. The European Chemicals Agency (ECHA) and U.S. FDA both put rigorous demands on traceability and impurity profiles. In global trade, shipments can hit roadblocks if paperwork gets sloppy or if a manufacturer’s facility hasn't passed recent audits.
From personal experience in small-molecule sourcing, unverified suppliers create more long-term costs than savings. It takes time and persistence to build relationships with vendors who truly understand the sensitivities of chiral intermediates like this one. Regular site audits, third-party sample checks, and keen attention to lot-to-lot variation always pay off—especially if your business can’t afford a surprise disruption or a product recall.
Cultivating a dependable channel for sizeable shipments of (R)-(+)-N-Boc-3-Pyrrolidinol means going beyond price comparison. Groups successful in the custom synthesis and contract manufacturing space recognize the value of partnering direct with facilities rather than just resellers. Competitive advantage often goes to those who lock in annual contracts or volume commitments, getting priority in scheduled runs and sometimes better payment terms. Some innovators even co-invest in facility upgrades or process improvements, ensuring both continuity and the right quality specs.
Another promising approach involves collaborative quality initiatives. Joint site visits, transfer of analytical methods, or even personnel exchanges can reduce communication gaps and foster transparency. It’s not just about getting material in a drum—it’s about knowing exactly what you’re putting into a product for global distribution.
Sourcing something like (R)-(+)-N-Boc-3-Pyrrolidinol at commercial scale takes both technical savvy and a honed partnership mindset. Fact-checking every shipment, keeping your compliance documentation airtight, and maintaining a dialogue with production chemists usually set the best teams apart. In today’s highly regulated environment, a grain of diligence prevents a mountain of problems down the road.
| Names | |
| Preferred IUPAC name | tert-butyl (R)-3-hydroxypyrrolidine-1-carboxylate |
| Other names |
(R)-(+)-N-tert-Butoxycarbonyl-3-pyrrolidinol (R)-3-Hydroxy-1-(tert-butoxycarbonyl)pyrrolidine (R)-(+)-Boc-3-Hydroxypyrrolidine |
| Pronunciation | /ɑːr pʌs ɛn bɒk θri pɪˈrɒlɪdɪnɒl/ |
| Identifiers | |
| CAS Number | “123332-60-1” |
| Beilstein Reference | 78457 |
| ChEBI | CHEBI:131997 |
| ChEMBL | CHEMBL415490 |
| ChemSpider | 118786 |
| DrugBank | DB08376 |
| ECHA InfoCard | 27-215-147-1 |
| EC Number | 276-100-4 |
| Gmelin Reference | 1374587 |
| KEGG | C19061 |
| MeSH | 3-Pyrrolidinol |
| PubChem CID | 10429492 |
| RTECS number | WH8041923 |
| UNII | DBB85W3P2G |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID9020452 |
| Properties | |
| Chemical formula | C9H17NO3 |
| Molar mass | 145.19 g/mol |
| Appearance | White solid |
| Density | 1.1 g/cm3 |
| Solubility in water | slightly soluble |
| log P | 0.00 |
| Acidity (pKa) | 14.5 |
| Basicity (pKb) | 6.31 |
| Magnetic susceptibility (χ) | -60.63×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.485 |
| Viscosity | Viscous oil |
| Dipole moment | 2.71 D |
| Thermochemistry | |
| Std enthalpy of combustion (ΔcH⦵298) | ΔcH⦵298 = -XXXX kJ·mol⁻¹ |
| Hazards | |
| Main hazards | Causes skin irritation, causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07, GHS08 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P264, P270, P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 144.1 °C |
| PEL (Permissible) | Not established |
| REL (Recommended) | 5 |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
N-Boc-3-Pyrrolidone 3-Pyrrolidinol (S)-(-)-N-Boc-3-Pyrrolidinol N-Boc-pyrrolidine N-Boc-4-Pyrrolidinol N-Cbz-3-Pyrrolidinol |