Piperidine rings have shaped modern synthetic and medicinal chemistry since the early days of pharmaceutical research. Adding a Boc (tert-butoxycarbonyl) group for N-protection was a game-changing move for project chemists who spent long hours wrestling with unstable amines and inefficient syntheses. The chiral (R)-3-Boc-Aminopiperidine sits on decades of incremental progress, with protecting groups like Boc gaining popularity in the 1970s. Groups racing to build selective receptor modulators and advanced biologically active compounds gravitated to reliable intermediates, and (R)-3-Boc-Aminopiperidine’s profile soon met those demands, especially after enantioselective synthesis made it accessible on meaningful scale in the early 2000s. Its story marks a shift from trial-and-error benchwork to structure-activity relationships and streamlined synthetic strategies, showing how organic chemistry matured alongside drug discovery.
Often described as a white or off-white crystalline powder, (R)-3-Boc-Aminopiperidine gets stored in brown glass under argon in most research labs. Its distinguishing feature is the Boc-protected amine sitting on a chiral piperidine ring, which allows selective deprotection and further transformation. Not many intermediates offer the trio of stability, chirality, and simple handling in routine organic synthesis. Every vial symbolizes not just a reagent, but a trusted building block for assembling more complex scaffolds in pharmaceutical and chemical biology research.
(R)-3-Boc-Aminopiperidine usually appears as fine granules or powder, with a melting point sitting around 70–75 °C. In a glovebox or standard lab setting, technicians note its minimal hygroscopicity—a blessing for those who’ve watched lesser reagents clump overnight. The molecule enjoys moderate solubility in common organic solvents like dichloromethane, acetonitrile, and methanol, but resists dissolution in water, which simplifies purification by extraction. The Boc-protected amine sports robust chemical stability under ambient conditions, but any seasoned chemist knows to keep strong acids and bases far from the workup flask until the protection step’s time arrives.
Commercial samples come labeled with the product’s CAS number (143900-44-1), molecular formula (C10H20N2O2), and molecular weight (200.28 g/mol). Purity typically exceeds 98%, and certificates of analysis log chiral purity, often over 99% ee, to guarantee stereochemical fidelity. Proper labeling calls out the chiral (R)-configuration and bulk lots carry batch-specific details on residual solvents, common byproducts, and date of manufacture. Laboratories keep safety sheets on file that summarize handling, storage, and disposal instructions.
Synthesis usually begins with chiral pool strategies or resolution of racemic mixtures. The most streamlined process starts from commercially available 3-aminopiperidine, using chiral auxiliaries or asymmetric hydrogenation to set the R-stereochemistry if optically pure starting material isn’t at hand. The Boc group gets introduced with di-tert-butyl dicarbonate (Boc2O) in the presence of a base, often triethylamine or sodium carbonate, under inert atmosphere. Purification by extraction and recrystallization gives the high-purity intermediate. As old chemists know, minimizing racemization during Boc protection means strict temperature control and quick workups.
Preparation sits at the start of a network of possible downstream chemistry. Researchers remove the Boc group selectively with trifluoroacetic acid or other strong acids, exposing the primary amine for further functionalization. Reductive alkylation, acylation, or installing various side chains can give rise to a library of analogs for screening. The piperidine ring tolerates a surprising range of transformations—from halogenation and oxidation to cyclizations that yield rigidified, drug-like molecules. For medicinal chemists, these modifications turn (R)-3-Boc-Aminopiperidine into more than a synthetic waypoint; it becomes a core around which they build drug candidates with improved potency and pharmacokinetics.
Ordering managers and bench chemists encounter several aliases on catalogs: (R)-N-Boc-3-aminopiperidine, (R)-tert-butoxycarbonyl-3-aminopiperidine, or occasionally the systematic mouthful (R)-1-(tert butoxycarbonyl)piperidin-3-amine. Each supplier brands it slightly differently, but those in the know spot the chiral notation straight away, distinguishing it from the S-enantiomer, which fits entirely different pharmacological niches. Both research-grade and GMP samples float through procurement systems under these names, depending on the regulatory context.
Those used to handling it recognize the mild, sometimes almond-like odor that hints at amines beneath the surface. Despite its relatively low toxicity, proper PPE still matters—nitrile gloves, goggles, and good ventilation cut the risk of sensitization or mild respiratory irritation. Spills get mopped up with basic absorbents, while waste heads out as hazardous organic residue. Facilities with high-throughput syntheses run routine GC or HPLC checks both to verify purity and to avoid cross-contamination with structures that can complicate downstream reactions or bioassays. Emergency protocols echo standard secondary amine guidelines: rinse skin, avoid inhalation, and keep incompatibles separated.
Drug discovery teams reach for (R)-3-Boc-Aminopiperidine in early library synthesis for CNS, oncology, and antiviral projects. The chiral amine slips easily into piperidine-based drug scaffolds, enabling rapid construction of analogs that help map biological activity. Researchers at biotech startups and large pharma organizations alike rely on it to build GPCR modulators, kinase inhibitors, and transporter ligands. Outside pharma, core modifications find use in material science, particularly for new polymers and functional materials, where the piperidine imparts chemical stability and reactivity not easily achieved by other nitrogen-containing rings.
Continuous process improvement defines current R&D trends. Newer asymmetric catalysts and biocatalytic approaches have started to replace resolution-based methods, improving both yield and environmental footprint. Teams explore greener solvents, automation-friendly protocols, and telescoped reactions that cut down on labor, waste, and cost. The basic framework has inspired derivatives with fluorine, alkyl, and heterocycle substituents, broadening its use in SAR (structure-activity relationship) studies. Grant applications now pitch ongoing work using its core to build libraries targeting undruggable proteins or allosteric enzyme sites.
Toxicology screens show relatively mild risk for acute or chronic exposure. Most cell assays report low cytotoxicity, though direct skin or eye contact occasionally causes mild irritation. Chronic administration studies in rodents flag minimal bioaccumulation or organ toxicity, yet regulatory filings call for careful handling, especially where metabolites might share mechanistic features with neuroactive or addictive compounds. Environmental fate studies note its tendency to degrade slowly in soil and water, mainly through hydrolysis and microbial breakdown, without forming persistent toxic products.
As demand climbs for chiral intermediates in drug development, the market for (R)-3-Boc-Aminopiperidine shows no sign of slowing. On the research front, machine learning–guided synthesis and flow chemistry have begun transforming how chemists design routes involving this compound, with automation poised to boost output for screening libraries. With regulatory trends pushing for greener and safer syntheses, companies invest in continuous process optimization and sustainable feedstocks. For those invested in medicinal chemistry, the structural backbone will only rise in value as next-gen drugs demand ever-more subtle modifications and precise stereochemistry. Bench scientists and manufacturers who keep pushing innovation around this small but crucial intermediate will drive forward both science and industry for years to come.
Chemistry has a knack for revealing how small tweaks in molecules end up creating big impacts in the real world. Take (R)-3-Boc-Aminopiperidine, for example. This name looks like it could scare off just about anyone outside a lab, but the story behind it speaks volumes about design, direction, and care in science.
(R)-3-Boc-Aminopiperidine starts with a piperidine ring. Imagine a hexagon of five carbon atoms and one nitrogen, like a circle you’d see in a middle school geometry class, only this one shows up in the guts of many medicines. Place an amino group at the third carbon and don’t forget the “(R)” part. That little letter adds only one extra twist—a specific three-dimensional arrangement, not just a flat drawing. Many chemicals have mirror twins, but only one works the way we need in drug discovery. Picking the right one keeps side effects out and boosts helpful action.
The Boc in the name means tert-butoxycarbonyl. Chemists love this group because it protects things. Add it to nitrogen, and suddenly that spot on the molecule won’t react with everything thrown its way. It’s like giving an overexcited part of the molecule earplugs so it keeps quiet until the real action starts. Imagine trying to paint only a portion of a fence: you might tape up the rest so you don’t get splatter everywhere. That’s the Boc group at work, masking and unmasking to get precision.
Put together, (R)-3-Boc-Aminopiperidine isn’t some abstract entity. Medicinal chemists hunt for scaffolds like this to build drugs that target pain, mental health, infections—you name it. They keep using rings and side chains like these because they fit certain parts of the body just right. Miss on the structure, and you might get nothing but a batch of chalk powder. Get it right, and suddenly you have a tool to start making a pill or a patch that helps real people get through the day.
Working with molecules like this doesn’t look as glamorous as TV shows might suggest. Synthesizing (R)-3-Boc-Aminopiperidine means juggling delicate steps, from protecting groups to purifying the product. Each stage brings tough decisions, like whether to keep things slow and careful or rush for yield. Many chemists learn quickly that messing up the order wastes both time and money, especially if the wrong version—or “enantiomer”—slips into the mix.
Back in college, synthesizing compounds that used a Boc group always meant spending half the afternoon waiting for reactions to finish and the other half hoping that nothing decomposed before purification. Seeing a clean NMR or IR spectrum at the end felt like a minor miracle some days. Talking to folks in pharma, the stakes only climb higher—one contaminant or wrong step, and a batch slated for testing gets tossed.
Better access to chiral chemicals like (R)-3-Boc-Aminopiperidine helps research keep moving. Automatic synthesizers and smarter purification tools lower errors, save resources, and cut down on trial-and-error. Making high-purity building blocks more affordable and easier to obtain means new therapies show up faster out in the world, helping more people instead of spending extra years on the bench.
Behind that name, there’s a combination of careful planning and relentless hard work, all leading to molecules that make a difference where it matters—in the clinic, in the pharmacy, in the lives of patients hoping for solutions.
Step into any synthetic chemistry lab these days and you’ll find shelves lined with compounds you may have never heard of. (R)-3-Boc-Aminopiperidine is one that always catches the eye, mainly for what it unlocks in the hunt for new medicines. The name might sound intimidating, but the work done with this chemical shapes how new drugs come together, especially those meant to nudge the nervous system in the right direction.
Drug developers reach for (R)-3-Boc-Aminopiperidine when scaffolding the backbone of something bigger—a potential medicine. Most of the value sits in the amine group, protected by the bulky Boc group. Researchers swap parts of this molecule like puzzle pieces, trying different combinations to change how test compounds behave. If you’re after a new antipsychotic, an antidepressant, or a way to relieve neuropathic pain, this amine comes up a lot. It finds a spot in drug candidates because it helps the molecule cross certain biological barriers and bind better to targets in the body.
One straightforward example: scientists often use this molecule as a key step when building piperidine rings—the sort that show up in a surprising number of modern medicines. The R-stereochemistry adds another layer of specificity, fine-tuning how potential drugs interact with the chemistry of the brain. It was eye-opening for me, early in my career, to see how meaningfully a single chiral center can influence a drug's activity and safety profile. Shave off the protective Boc group, link in a new fragment, and the process repeats until something promising emerges.
Beyond making medicine, chemists also use (R)-3-Boc-Aminopiperidine for custom-made molecules used in agricultural chemicals, materials science, or even dyes. The protected amine structure is like a temporary shield—easy to snap off under mild conditions—so the sensitive amine doesn’t get damaged during tough chemical reactions. In practice, it keeps the molecule clean and easy to manipulate, which saves effort and materials.
The chiral angle shouldn’t be overlooked. Many natural and synthetic compounds come in right-handed and left-handed forms, with only one form showing the effect you want (and sometimes the other doing harm). The R-form allows for more defined experiments and more targeted results, which is why research teams pay extra for enantiomerically pure reagents.
For all its benefits, sourcing (R)-3-Boc-Aminopiperidine can bring headaches. Price jumps, slow deliveries, or questions about purity complicate life for any lab on a tight budget. I’ve seen researchers stuck in limbo, project timelines stretched because of unreliable suppliers. This says something broader about the supply chain for specialty chemicals—global shocks or policy changes can put a pinch on scientific progress.
Getting around this means building stronger ties with vendors, pooling orders with other groups, or sparking interest in local production. Introducing more checks and transparency on reagent quality would keep labs from betting on a batch only to find it falls short. Automated monitoring or digital tracking of chemicals through the research pipeline might also help dodge supply snags before they turn into real problems.
From research experience, I’ve learned that new chemistry rarely happens in a vacuum. A single molecule like (R)-3-Boc-Aminopiperidine sets a ripple effect, influencing dozens of early drug candidates, agricultural agents, and even teaching laboratories. Making sure researchers have reliable, pure compound means every other step they take stands on steadier ground.
Good chemistry starts with clean building blocks. In pharmaceutical labs, (R)-3-Boc-Aminopiperidine plays this role. The catch? Purity calls the shots on how well this molecule does its job. Generally, a purity of 98% or better appears as the rule if you peek at what trusted suppliers put on their documentation. Chemists I know even push for 99% where quality standards grow tighter, especially when folks use the compound for active drug synthesis or studies involving strict regulatory expectations.
Impurities don’t always announce themselves. Some sneak in quietly, hanging around from earlier steps, or pop up as things break down during handling. You can see their fingerprints on your results—unexpected peaks in HPLC, headaches in downstream reactions, and even batch failures. In one project, an impurity under one percent scrambled a weeks-long experiment and forced us to retrace every step. Purity isn’t a paperwork box to tick; it literally saves time and sanity.
In chemical sourcing, specifications offer a guarantee. The standard for (R)-3-Boc-Aminopiperidine will read “≥98%” on almost any professional data sheet, measured by HPLC or GC. For niche sectors, including biotech or clinical research, scientists push higher. Recrystallization, careful storage, and analytical proof build that confidence. I’ve known more than one researcher willing to reject an entire batch over a 0.2% blip because they knew how quickly things can go sideways in a finely-tuned process. That attention to detail often separates average results from breakthroughs.
The difference between 95% and 99% doesn’t always jump out at first. Yet if you spend time in molecule assembly—building peptides or new APIs—those missing percentage points often mean stubborn by-products or inconsistent yields. Analytical chemistry gives the tools to really “see” purity. Chromatography, NMR, even melting points tell their story if you listen. For folks crafting high-value molecules, even a sliver of the wrong material can mean unexpected toxicity, side reactions, or regulatory failure.
Experienced researchers don’t trust labels alone. If stakes run high, samples get retested in-house before they touch core chemistry. Taking a little powder, running it through HPLC or LC-MS, and comparing those results to supplier sheets builds trust. Reliable supply chains, repeat documentation, and solid analytical skills let chemists breathe easier. I once saw a team halt work for two weeks rather than rush with an untested new lot, simply because they’d burned their fingers on contaminated stock years before.
Working with (R)-3-Boc-Aminopiperidine reminds everyone how much the basics matter. Suppliers who show full data—chromatograms, certificates, batch numbers—clear a lot of hurdles early. Lab teams that always check a sample themselves dodge surprises, and open conversation with vendors helps flag issues early. As the expectations on research keep climbing, meeting or beating that 98% purity mark ceases to be a luxury. It turns into the table stake for safe, efficient, and honest science.
On a regular workday, shelves crowd with glass bottles, powders, and odd smells—typical for anyone in a chemistry lab. Sometimes, the job means handling sensitive compounds like (R)-3-Boc-Aminopiperidine. Let that bottle sit in the wrong spot, and results can turn questionable fast. It takes only one careless placement for months of careful planning to unravel.
Every chemist learns the hard way—water finds its way in where you least want it. (R)-3-Boc-Aminopiperidine draws moisture from the air, and that can change its properties. I remember opening a seemingly dry bottle, only to get a sticky mess inside. Labs lose time and money cleaning up or testing gummed-up material. Dry, airtight containers with snug lids cut down that pain. Adding a silica packet or two into the storage jar helps keep it all bone dry.
A stuffy, overheated storage room always spells trouble. The compound loses punch if the mercury creeps above 25°C. Cool, shaded storage—not stuck next to a sunny window or under glaring lights—keeps the material from breaking down. I’ve seen temperature logs; one hot stretch throws off entire batches. Small dedicated fridges pay off by locking in the structure and making repeat synthesis more predictable.
It’s tempting to grab a clear bottle for easier tracking, but light creeps in and speeds up degradation. I’ve worked in places where yellow-brown vials line the shelves, and for a reason. Amber glass works best. Even wrapping bottles in foil can help. By blocking sunlight and harsh fluorescent light, the compound keeps its structure intact longer.
Faded tape, cryptic handwriting—both waste time, and one mix-up can burn through weeks of work. Date every bottle. List purity and batch number. In my own notebooks, I always jot down the source and last inspection date. Clear notes stop confusion, especially after turnover or during audits, and help everyone trace a bad lot back to its source.
There’s never a shortage of gloves and goggles in any half-decent chemistry space. (R)-3-Boc-Aminopiperidine may not look dangerous at a glance, but breathing in powder or touching without protection can add up health risks. Once, a slight breeze scattered a puff across our workbench—it only took a split second to learn respect for caution. Handle only under a fume hood or ventilated space, and wash up after, every time.
Old bottles clutter valuable storage and tempt risky use of degraded material. Quarterly cleanouts keep the stash lean and safe. I’ve found surprise leftovers that made it past memory and inventory sheets. Disposing of outdated or questionable product using recommended chemical waste procedures saves labs from trouble and fines.
Every detail, from where the bottle sits to how it’s sealed, builds a chain of reliability for (R)-3-Boc-Aminopiperidine. Getting it right helps scientists from grad students to pharma pros avoid simple mistakes that can grow into headaches later. In the end, a little extra care pays back every day a clean, dry, fresh batch delivers the results you expect.
Anybody working in a chemistry lab knows the challenge of finding chemicals in the right amount, not just the right quality. With (R)-3-Boc-Aminopiperidine, that challenge feels familiar. It's a specialty building block for making pharmaceuticals, so you don’t always buy it by the barrel. Standard suppliers usually offer three basic package sizes: one gram, five grams, and twenty-five grams.
These sizes aren’t random. From my own ordering headaches, the one gram vial works best in research settings, where someone tests out a new synthetic pathway or screens a few reactions. Not everyone wants a closet full of half-used bottles, especially when shelf life matters and budgets always feel tight.
For chemists scaling up their experiments, the five gram bottle becomes gold. This size handles pilot batches and repeat procedures. In my old university lab, our project always landed right in this range. Nobody likes cutting open a fresh bottle for every trial, but purchasing huge amounts costs more money than the project earns. Five grams balances price with convenience.
Bulk buyers usually go for twenty-five grams or more. At this level, the chemical supports early scale-up work—think preclinical batches, or perhaps the prep room at a drug discovery startup. Nobody wants to waste time and money splitting smaller vials or dealing with backorders. Here, one order keeps the workflow moving with fewer interruptions.
Big pharmaceutical labs hit a problem. Twenty-five grams won’t last long on a continuous run. The better chemical suppliers have started taking requests for larger, custom sizes, from 100 grams up to a kilo or more. You’ll see this in online order forms, where the supplier asks you to send a quote request for anything over a standard amount.
I’ve seen this first-hand: you call or email, and a rep comes back with a lead time and price. They can change bottle size, offer different packaging materials, or even split quantities over multiple containers for easier handling.
There’s a story behind each size on that list. Small amounts let startups take risks without overcommitting cash. Midsize packages meet day-to-day research. Large quantities benefit from price breaks and good supply relationships. The bottom line: the “right” size depends on your workflow. An undergraduate running a handful of reactions simply doesn’t need a kilo, but a drug company might run through several kilos a month.
Chemicals like (R)-3-Boc-Aminopiperidine need thoughtful packaging because they don’t last forever. Glass bottles tend to be the default for research packs, since piperidine derivatives can attack the wrong kinds of plastic over time. For bigger jobs, some suppliers offer lined HDPE jugs meant to handle both the chemical and rougher shipping conditions. Nobody wants to open a box and find powder everywhere instead of neatly sealed bottles.
If you don’t see the size you need, reaching out matters. Suppliers have flexibility built into their shipping lines, partly because so many buyers ask for special amounts. It helps to talk up front. That’s saved me money on projects that only needed a midsize batch, and it’s stopped colleagues from ordering way more than they could use in a year.
Staying practical about package sizes for (R)-3-Boc-Aminopiperidine just means less waste, easier workflow, and a smoother research process. That’s something any chemist will appreciate, whether in a university lab or an industry plant.
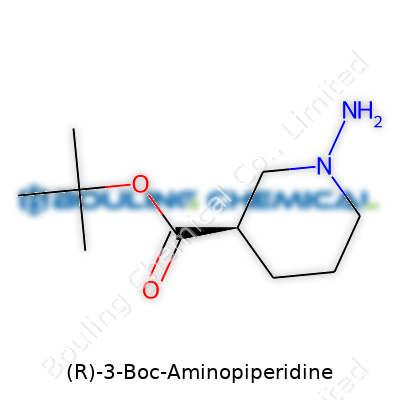
| Names | |
| Preferred IUPAC name | (3R)-1-tert-butoxycarbonylpiperidin-3-amine |
| Other names |
tert-Butyl (R)-piperidin-3-ylcarbamate (R)-3-(Boc-amino)piperidine (R)-N-Boc-3-aminopiperidine (R)-3-aminopiperidine, N-Boc protected (R)-tert-butoxycarbonyl-3-aminopiperidine |
| Pronunciation | /ɑːr θriː bɒk əˈmiːnoʊ pɪˈpɛrɪdiːn/ |
| Identifiers | |
| CAS Number | 141699-58-5 |
| 3D model (JSmol) | `3Dmol.js:load=inline "data/mol;base64,C1CNCCC1N.C(C)(C)OC(=O)"` |
| Beilstein Reference | 12665884 |
| ChEBI | CHEBI:131279 |
| ChEMBL | CHEMBL1099290 |
| ChemSpider | 13284235 |
| DrugBank | DB08398 |
| ECHA InfoCard | 03ca597b-cf47-4b04-92f6-c9ca03410835 |
| EC Number | NA |
| Gmelin Reference | 1542066 |
| KEGG | C18973 |
| MeSH | D000068640 |
| PubChem CID | 123627433 |
| RTECS number | UJ6423000 |
| UNII | QIT6K2H98A |
| UN number | Not regulated |
| CompTox Dashboard (EPA) | DTXSID10443670 |
| Properties | |
| Chemical formula | C10H20N2O2 |
| Molar mass | *N 3 B o c - A m i n o p i p e r i d i n e * : 2 0 0 . 2 9 |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.1 g/cm³ |
| Solubility in water | Insoluble in water |
| log P | 1.18 |
| Acidity (pKa) | pKa ≈ 10.7 |
| Basicity (pKb) | 3.3 |
| Magnetic susceptibility (χ) | -67.0e-6 cm³/mol |
| Refractive index (nD) | 1.485 |
| Dipole moment | 3.13 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 308.6 J·mol⁻¹·K⁻¹ |
| Hazards | |
| Main hazards | May cause respiratory irritation; causes skin irritation; causes serious eye irritation. |
| GHS labelling | GHS07, Exclamation Mark, Warning, H302, H315, H319, P261, P264, P270, P305+P351+P338, P337+P313 |
| Pictograms | GHS07, GHS05 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P332+P313, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > 113.7 °C |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/mL DMSO |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
N-Boc-piperidine 3-Aminopiperidine 3-Boc-aminopiperidine (S)-3-Boc-aminopiperidine 1-Boc-piperidine-3-amine |