Decades ago, chemists chasing new intermediates for drug discovery recognized the need for stable, modifiable building blocks that could open doors in synthetic routes. Piperidine derivatives earned their stripes through pharmaceutical development in the 20th century, driven by a hunger for selectivity and fidelity in molecular design. The (R)-1-Tert-Butyloxycarbonyl-3-Hydroxypiperidine molecule arrived as a solution for chiral intermediates, letting researchers sidestep the pitfalls of racemic mixtures. The Boc-protecting group grew popular in peptide synthesis, adding another tool for manipulation without unwanted side reactions. Laboratories across Europe and the US soon adopted this approach to streamline synthesis steps, refine purification, and minimize wastes—saving time, money, and raw material during the search for new drugs.
A bottle of (R)-1-Tert-Butyloxycarbonyl-3-Hydroxypiperidine might look unremarkable: a white or off-white crystalline solid tucked away in amber glass. For medicinal chemists and process engineers, this is a smart intermediate that handles functional group protection with grace. Pairing a piperidine core with hydroxy and Boc groups brings two key handles: ready access for further derivatization and improved control during stepwise synthesis. The chiral purity of the (R)-enantiomer means downstream reactions benefit from stereoselectivity, a backbone for safer and more effective pharmaceutical compounds.
Clear, reliable data shape the daily workflow in chemical plants and academic settings. This compound brings a melting point in the range of 97-101°C, good shelf stability, and moderate solubility in common organic solvents—think dichloromethane, ethyl acetate, or even methanol. As a solid, it travels well between storage and reaction vessels and resists moisture and oxygen when kept sealed. In my own experience working with similar Boc-protected amines, the smell is subtle, the color consistent, and storage under argon or nitrogen prevents slow degradation. Sensitive NMR analysis and chiral HPLC testing reveal a clean signal profile when handled with dry glassware, key for compliance and yield predictions.
The modern supply chain runs on clarity and precision. Labels for this chemical detail enantiomeric excess (EE, rarely less than 98%), purity (typically 98%+ by HPLC), CAS number (143900-44-1), and UN hazard codes for shipping. Safety Data Sheets (SDS) accompany every shipment, specifying storage temperature, stability data, and recommendations for disposal. Lot-specific certificates provide reassurance about trace impurities, chiral purity, and water content. Customers in regulated industries—pharma, biotechnology, advanced polymers—expect this level of transparency before committing a single gram to a process.
Crafting this compound generally calls for a two- or three-step synthesis, starting from a suitable piperidine base. The hydroxyl group is introduced via dihydroxylation or direct substitution, using established asymmetric methods to generate the desired chirality. Protecting the amine comes next, with di-tert-butyl dicarbonate (Boc2O) and a mild base, preserving the configuration through careful temperature control. Column chromatography or crystallization purifies the intermediate. Over the years, green chemistry practices have crept into these steps, swapping harsh reagents for milder conditions and recycling solvents wherever possible—an everyday reminder that scalable chemistry and sustainability should partner up.
For me, the true value lies in what follows: the hydroxy and Boc groups act as twin switchboards, letting researchers make subtle or bold changes to the piperidine core. Deprotecting the Boc group cues up coupling reactions or ring closures, favored by peptide chemists building complex molecules. The hydroxy can undergo acylation, alkylation, or oxidation, feeding downstream modifications for drug analogs or probe molecules. Each step opens opportunities for tuning pharmacokinetic and pharmacodynamic profiles. Whether it’s developing beta-lactamase inhibitors or new opioid antagonists, the flexibility of this intermediate echoes progress in medicinal chemistry.
Another layer of complexity comes from the way suppliers and researchers refer to this compound. Names like (R)-Boc-3-hydroxypiperidine, Boc-(R)-3-hydroxypiperidine, or (R)-N-Boc-3-hydroxypiperidin-1-ol show up in catalogs. CAS 143900-44-1 ties them together—something I check before confirming an order or running a reaction. Confusion can creep in without this diligence; a slip in nomenclature may result in delays or costly mix-ups, especially in busy research environments juggling multiple chiral intermediates.
No chemical leaves a reputable warehouse without safety standards. The compound isn’t listed as especially hazardous, but gloves, lab coats, and safety glasses stay on during handling. Standard ventilation keeps dust and fumes at bay, a rule reinforced by years keeping hood spaces tidy. Spills and exposure call for prompt cleanup and washing due to mild irritation risk. Accurate logs track storage and use to prevent expired material from entering key syntheses, building a culture where good habits prevent serious slip-ups. Disposal aligns with established protocols for non-chlorinated organic solids, minimizing environmental risk.
Medicinal chemistry teams pull (R)-1-Tert-Butyloxycarbonyl-3-Hydroxypiperidine from the shelf for a wide array of research campaigns. Its performance as a chiral building block streamlines the creation of HIV treatments, central nervous system drugs, and enzyme inhibitors. Fine-tuning molecular frameworks for selectivity and metabolism starts here. As demand for targeted therapies and rapid drug prototyping rises, intermediates like this one prevent bottlenecks in lead optimization. Polymer scientists dip into the piperidine toolkit to design advanced materials where chirality and hydroxy functions create new physical behaviors. Each project draws from an ever-growing playbook of synthetic possibilities.
Academic and industrial R&D units probe into new synthetic routes for cleaner, faster, scalable production of this intermediate. I’ve seen teams automate route scouting with flow chemistry rigs, slashing solvent use and reaction times. Funding agencies look closely at sustainable pathways; every new publication adds to collective know-how. Collaborations between universities and small biotech firms have sped up the optimization process, using AI-driven retrosynthesis tools to suggest shortcuts or alternative catalysts. These investments trickle down to more robust supply chains and affordable starting materials for smaller labs and startups, opening doors for more discovery work.
Diving into toxicity, not much stands out in the literature, but gaps remain. Acute oral and dermal studies in mammals suggest minimal risk at low exposure levels; the real danger comes from chronic inhalation or eye contact. Some off-target effects linked to piperidine rings—such as mild neurological response or liver enzyme modulation—deserve closer monitoring during long-term handling. Regulatory filings require traceability and full panel screening. Instilling respect for analytical controls and meticulous recordkeeping keeps both staff and final drug candidates on the right side of safety thresholds, especially when unknowns could halt clinical trials downstream.
Looking ahead, the future for (R)-1-Tert-Butyloxycarbonyl-3-Hydroxypiperidine pivots around accelerated drug development and green chemistry improvements. Demand for enantiopure intermediates won’t slow as personalized medicine and biocatalysis reshape pipelines. Process engineers hunt for cost-effective, eco-friendly manufacturing, moving away from rare metals and high-waste processes. Automation and real-time process analytics are sneaking into everyday practice, fueling higher throughput and reliability. Expanded toxicity screens, improved catalysts, and alternative protecting groups promise to widen the chemical’s usability even further, promising new therapeutics and smarter materials in ever-tightening research budgets.
Chemists rarely talk about a compound like (R)-1-Tert-Butyloxycarbonyl-3-Hydroxypiperidine without bringing up purity. It affects everything from downstream reactions to final product safety. Quality checks and reproducibility in pharmaceutical labs turn on detailed measurements. If you miss the mark on purity, you risk wasted time, false negatives, and batch recalls. That kind of setback hurts researchers and, more importantly, the patients waiting on results.
Suppliers list purity percentages to reassure buyers, but context gives those numbers weight. Big pharma typically aims for ≥98% pure starting materials. In research, some run work with 95% pure intermediates, but nearly everyone demands stricter standards from anything that reaches clinical phases. Contaminants, byproducts, and leftover reagents signal shortcuts or breakdowns in quality controls. Chiral purity raises another bar, especially because (R)-1-Tert-Butyloxycarbonyl-3-Hydroxypiperidine serves as a key intermediate for chiral drugs.
Companies like Sigma-Aldrich or TCI usually advertise purity figures right next to the product listing—often at 97% or 98%. I’ve seen fellow researchers argue over a single percent since it can mean hours of extra purification. A few NMR peaks or a weird HPLC baseline can set off a day of troubleshooting. Nobody in a synthetic lab wants to find surprise masses or impurities on their LC-MS report at the last step. Every point under 98% means time, money, and uncertainty.
Purity doesn’t depend just on skill; it reflects raw material source, batch size, and how much the supplier invested in post-synthesis cleanup. If a supplier pushes out large volumes fast, purity can drop. Small-batch, high-purity synthesis costs more but cuts down on unwanted side-products. Crude product from unoptimized routes leads to more byproducts. Even solvents and glassware make a difference—cross-contamination once turned a run in my old lab from 99% pure to 90%. That mishap hammered in the lesson: don’t rush basic prep work, especially not with chiral intermediates like this one.
Low-purity intermediates bring unexpected headaches. Impurities stall reactions, poison catalysts, or create unidentifiable byproducts. Synthesis protocols can cascade into chaos. Chiral purity is another beast—minor changes can flip selectivity in enantioselective synthesis. I’ve watched teams lose weeks hunting down sources of contamination in batches. If pharmaceutical compounds contain unwanted stereoisomers, clinical candidates may fail to meet regulatory standards.
Proper purchasing saves stress. Trusted suppliers provide certificates of analysis with every lot, listing everything from percent purity to water content and even enantiomeric excess. Independent labs double-check these values with NMR, IR, and chromatography before using a new batch. For extra assurance, some labs purify even more in-house using flash chromatography or recrystallization. This hands-on approach keeps results reproducible and safe down the line. I’ve also seen teams split orders across suppliers, then sample and test each lot before scaling up.
More transparency draws a line between reliable vendors and those cutting corners. Open sharing of analytic data, details on synthetic routes, and even batch chromatograms can change the game. Tighter supply agreements, clear communication, and routine audits nudge everyone toward higher purity.
Purity calls for sweat, vigilance, and honest analysis. Skipping steps or trusting a number on a label without verification bites every time.
(R)-1-Tert-Butyloxycarbonyl-3-Hydroxypiperidine steps out of a niche organic chemistry textbook and straight into the lab where small missteps can cost time and money. Ask anyone who’s handled delicate chemical intermediates—they don’t let just any molecule lounge around on the benchtop all day. This one shows sensitivity to air and moisture, which makes proper storage less an academic suggestion and more a requirement if you want reliable results.
I remember a postdoc at my old lab agonizing over murky NMR spectra after just a weekend. He traced the problem to a supposedly “stable” batch of piperidine derivative that had been left at room temperature and had pulled water from the air. The stuff ended up partially hydrolyzed, and the cost wasn’t just wasted material—it set him back a week in synthesis steps. Ambient humidity and visible light often cause these sorts of headaches. No special instincts required; just a decent appreciation for what a lid and a fridge can achieve.
So what works? First, a factory-sealed amber glass bottle takes priority over plastic. Glass keeps most chemicals from reacting and blocks light reasonably well. You stick that bottle in a cool, dry spot—most labs rely on refrigerators at about 4°C. Forget the old wine fridge, though. I’ve seen someone’s “dedicated” chemical fridge full of drinks and sandwiches contaminate an entire shelf of neat compounds. Food stays out, chemicals stay in, and clean labels save a world of confusion.
Desiccants keep the moisture at bay. Silica gel packets often ride along in the same drawer as small bottle batches. There’s something satisfying about opening a dry bottle and not catching even a hint of dampness. Plus, a well-fitted cap with a tight seal slows oxygen and vapor exchange, reducing the risk of slow, insidious degradation that doesn’t always show up until you check purity after a few weeks.
These habits don’t come from picky regulations—they're lessons learned the hard way. Degraded starting material torpedoes yield and purity. Monitoring temp and humidity cuts surprises during synthesis. Even a few degrees warmer storage shortens shelf life; crystals, clumps or discoloration point to bigger headaches if left unchecked. Just ask anyone who’s repeated a failed reaction and only later realized last month’s mistake snowballed down the line.
Technology sometimes helps. Remote data loggers track temperature swings now, so if the fridge fails over a holiday, someone gets a warning ping. Automation keeps human forgetfulness from ruining a promising project. Shared lab reminders and checklists for routine inventory checks give everyone accountability, not just the one postdoc whose project depends on this compound.
Tighter supply chain practices add another layer. Sourcing from reliable vendors with transparent shipment packaging and prompt handling on arrival means spending less effort cleaning up after degraded stock. Sometimes it pays to order a smaller batch, use it quickly, and avoid months of uncertain storage altogether. That sense of relief you get knowing that your bottle hasn’t been quietly decaying in a dusty back shelf? It never gets old. People start trusting results, and the lab feels a bit less like a gamble.
Storage slips in chemical labs often boil down to habits. Amber bottles, dry conditions, cool storage away from direct sunlight—none of it feels high-tech. Still, the difference becomes obvious as soon as things go right—and disasters stay rare. Old-school attention to detail makes a bigger impact than most fancy gadgets. It’s more than a checklist; it’s what keeps science moving one clean, sharp reaction at a time.
Digging through chemical catalogs will show just how much jargon and confusion swirl around compound names. One molecule can wear a dozen different names depending on the context, supplier, or how the researcher prefers to call it. The (R)-1-Tert-Butyloxycarbonyl-3-Hydroxypiperidine is a perfect example—its full IUPAC name is a mouthful, and searching by the casual or shorthand names can lead to trouble. That's where a CAS number steps in as a north star.
(R)-1-Tert-Butyloxycarbonyl-3-Hydroxypiperidine carries the CAS number 143900-44-1. With more than 200 million chemicals on record, putting a single, unique fingerprint on each substance is essential. In my own work ordering research intermediates, I’ve seen how easy it is to buy the wrong compound based on similar names. A CAS number saves time, money, and a whole lot of headaches.
Just a few digits make the difference between hitting the mark and chasing the wrong rabbit. Let’s say you’re working on a custom peptide synthesis or hunting for a specific enantiomer in the lab. The accuracy of the compound matters. One wrong variant, and the whole plan collapses. I’ve learned to always double-check the CAS number before approving an order. Experience has taught me that the lab drama that follows a misidentified chemical usually means delayed deadlines and wasted budgets.
Danger comes not only from getting the right molecule, but also in managing regulatory paperwork. Regulatory agencies track chemicals by their CAS numbers. This record becomes essential when reporting hazardous materials, writing up safety data sheets, or making sure nothing slips through the compliance cracks. Labs have strict files for every substance, all lined up by that little string of digits. You can wipe out guesswork with clear labeling, accurate tracking, and digital inventory tied right to the CAS number.
In the real world, people will misspell a compound name or grab a synonym off an old protocol. Relying on CAS numbers helps train new lab staff, supply teams, and even external auditors to speak a common language. I’ve watched junior chemists save a project simply by cross-checking a label with the catalog number, nipping a problem in the bud. Consistent use keeps confusion to a minimum and helps avoid dangerous mix-ups. Teaching the habit early turns into a reliable skill, just like always labeling your glassware or signing your lab notes.
Suppliers, buyers, and researchers have an interest in smoother transactions. Listing the correct CAS number—143900-44-1 for (R)-1-Tert-Butyloxycarbonyl-3-Hydroxypiperidine—prevents delays and lets people work with confidence. Most digital inventory software now lets you search and filter by CAS, which speeds up audits and makes regulatory reporting less of a chore. People build better habits when the right tool is just a search away.
Walking into a supplement shop, scrolling through endless protein powders, browsing hemp oils online—every package promises the moon. Shiny labels and buzzwords fight for attention, but most shoppers just want one thing: proof that what's on the label is what’s in the bottle.
A certificate of analysis (CoA) offers a window beyond marketing. It’s a printout with numbers—heavy metals, pesticides, strength, microbiology—almost unreadable at first. But that document means more than a sales pitch. It's a promise from the company that a third-party lab checked the product.
People have good reason to ask for a CoA. Last year, the FDA recalled dozens of supplements after tests revealed unlisted prescription drugs and too much lead in some batches. A scary thought, especially after a doctor tells someone to watch their health, or when parents buy baby formula. Past mistakes stick. It only takes one contaminated batch to shatter trust for years.
The supplement and natural product world exploded—thousands of companies now jump into the market, often using contract manufacturing. Quality changes from one batch to another. The CoA becomes a line of defense, separating a careful maker from someone just after quick profits.
Old friends have asked me how to pick a CBD oil or a protein powder safely. It comes down to demanding proof. We don't need detailed chemical knowledge—just the basics: Was it tested? Did it pass? Any batch with a proper CoA means the manufacturer isn’t hiding behind marketing fluff.
Plenty of companies don’t display CoAs, or they list a sample certificate that's months old, covering a past batch. Some claim “proprietary” information, or drag their feet when customers ask. These aren’t good signs. If I have to chase basic safety information, alarm bells go off.
I’ve dealt with brands that act defensive about testing. Usually, the excuse starts to unravel after a few simple questions. Sometimes, they’ve never done the tests. Or their supply chain is a black hole even for them. All this spells one reality—no reliable proof, no real trust.
There’s a solution, and everyone wins when companies take it: post full, batch-specific CoAs right on the product page--not after an email, not hidden in a corner. Show what’s in the jar and what’s not. Service isn’t just about smiling logos, it's about sharing tough data.
For shoppers, the habit is simple. Ask for the CoA early. If a company can't provide it, shopping somewhere else sends the right signal. For makers, owning the supply chain with full transparency sets some apart in a crowded world. Laboratories, third-party verification, clear numbers—this is the language of trust.
Real safety doesn’t hide behind jargon or fancy names. Clarity builds loyalty. In a market flooded with claims and counterclaims, those numbers tell the truth. That’s something worth more than a made-up guarantee.
Step into any modern synthetic chemistry lab and you’ll catch whispers about certain specialty molecules that zip from benchtop to bench notebook again and again. (R)-1-Tert-Butyloxycarbonyl-3-Hydroxypiperidine sounds like a mouthful, but it’s one of those building blocks researchers reach for in the dash to make next-generation drugs, especially in early-stage pharmaceutical design. The “Boc-protected” piperidine isn’t just an oddball—it solves sticky problems for chemists as they try to sketch out molecular blueprints that no one’s drawn before.
In hands-on chemistry, certain protections—literal ones, like the tert-butyloxycarbonyl (Boc) group—keep molecules from reacting with the wrong partners too soon. While chasing down a potential new treatment, a scientist will often “mask” a reactive spot to focus reactions exactly where they want them. The Boc group locks down the piperidine’s nitrogen. This opens the door to fiddling with the hydroxyl at carbon 3, stitching on new pieces, or bending the molecule into unfamiliar shapes.
Once the rest of the molecular framework is in place, that Boc group pops off easily with acids, releasing the original amine without damaging sensitive fragments nearby. In practice, this sidestep cuts down headaches during the dance of multi-step synthesis.
Walk through a research pipeline, and you’ll notice (R)-1-Tert-Butyloxycarbonyl-3-Hydroxypiperidine showing up as a “chiral intermediate.” The handedness (the “R” at the start) means something in medicine: your body treats left and right-handed molecules completely differently. Drug makers have learned to seek out molecules with specific chirality to tune safety and effectiveness. This little piperidine helps them build the backbone for antipsychotics, antivirals, anti-blood pressure drugs, and even experimental therapies against rare diseases.
I’ve seen research teams fight frustration trying to stitch together complex molecules, only to find that a reliable chiral piperidine—like this one—shaves weeks off their project. A few catalog suppliers can deliver it with the kind of purity and documentation you need for regulatory filings, which makes a big difference once development leaves the lab and moves toward trials.
For anyone scaling up, shelves start to groan under the weight of reagents, solvents, and jars with names most folks can’t pronounce. Yet producing this compound remains tricky. Its value, in part, stems from the technical slog it takes to make it right—settling the stereochemistry, keeping everything pure, and avoiding contamination that could upend a synthesis route.
Some small-scale research teams jump straight to custom synthesis services just to get enough for their experiments. This tells you something about demand and how even an intermediate—never actually meant to heal pain or fight infection—plays a huge role in launching medical breakthroughs.
The world of specialty chemicals often gets hung up on supply, cost, and safety. Not every lab can buy expensive chiral intermediates, so more efficient routes or greener synthesis methods would help. Cheaper, less hazardous production could let university teams explore more molecular options, fueling fresh ideas beyond the constraints of tight research budgets.
Stronger partnerships between chemical suppliers, start-ups, and academic labs might knock down some of those old barriers. As new techniques for asymmetric catalysis trickle out of the journals, the hope is that one day molecules like (R)-1-Tert-Butyloxycarbonyl-3-Hydroxypiperidine become less of a bottleneck—and more of a common tool to open up new chemical possibilities.
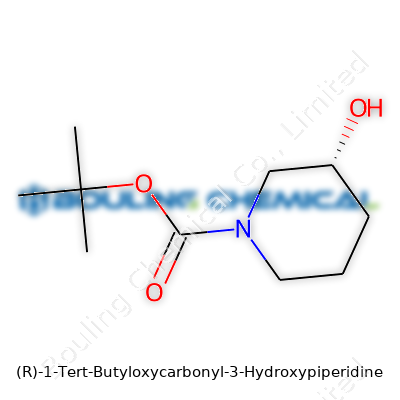
| Names | |
| Preferred IUPAC name | (3R)-1-(tert-butoxycarbonyl)piperidin-3-ol |
| Other names |
(R)-Boc-3-hydroxypiperidine tert-Butyl (R)-3-hydroxypiperidine-1-carboxylate Boc-(R)-3-hydroxypiperidine (R)-3-Hydroxy-1-(tert-butoxycarbonyl)piperidine |
| Pronunciation | /ɑːr wʌn tɜːrt ˌbjuːtɪlˌɒksiˈkɑːbənɪl θriː haɪˈdrɒksi pɪˈpɛrɪdiːn/ |
| Identifiers | |
| CAS Number | 143900-43-4 |
| 3D model (JSmol) | ``` /usr/local/jmol-14.31.54/jsmol/php/jsmol.php?id=1&model=_model&id_1&pdb=1&mol=(R)-1-Tert-Butyloxycarbonyl-3-Hydroxypiperidine ``` |
| Beilstein Reference | 120388-18-9 |
| ChEBI | CHEBI:187441 |
| ChEMBL | CHEMBL1276728 |
| ChemSpider | 22453807 |
| DrugBank | DB08363 |
| ECHA InfoCard | ECHA InfoCard: 1008909 |
| EC Number | 1343086-81-4 |
| Gmelin Reference | 106438-55-1 |
| KEGG | C14337 |
| MeSH | D017964 |
| PubChem CID | 10424451 |
| RTECS number | VA0655264 |
| UNII | 7A16B4AK5D |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C10H19NO3 |
| Molar mass | 189.26 g/mol |
| Appearance | White solid |
| Density | 1.1 g/cm3 |
| Solubility in water | Insoluble in water |
| log P | 0.0 |
| Vapor pressure | 9.01E-7 mmHg at 25°C |
| Acidity (pKa) | pKa ≈ 9–10 |
| Basicity (pKb) | 5.86 |
| Refractive index (nD) | 1.480 |
| Dipole moment | 3.05 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 387.6 J·mol⁻¹·K⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P305+P351+P338 |
| NFPA 704 (fire diamond) | 1-1-0 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 50 mg / 25mL |
| IDLH (Immediate danger) | NIOSH has not established an IDLH value for (R)-1-Tert-Butyloxycarbonyl-3-Hydroxypiperidine. |
| Related compounds | |
| Related compounds |
1-Tert-Butyloxycarbonyl-3-hydroxypiperidine 3-Hydroxypiperidine (S)-1-Tert-Butyloxycarbonyl-3-hydroxypiperidine Piperidine Boc-protected piperidine 1-Boc-piperidine |