Chemists in the latter half of the twentieth century started exploring the boundaries of protected amine chemistry as they hunted for ways to simplify complex syntheses. (R)-1-Tert-Butyloxycarbonyl-3-aminopiperidine, often referred by its shorthand Boc-3-aminopiperidine, came out of that wave of innovation. This molecule’s story runs parallel to the growth of peptide chemistry. Researchers kept bumping into issues with free amine groups reacting when and where they weren't supposed to, so the introduction of the Boc protecting group seemed like a key piece of the puzzle. Over time, not only peptide chemists but anyone working with functionalized piperidines started reaching for Boc protection as a reliable way to keep intermediates clean.
(R)-1-Tert-Butyloxycarbonyl-3-aminopiperidine typically hits the market as a crystalline white powder or sometimes in the form of a solid that melts just below 100°C. As a building block in organic synthesis, its value sits in the combination of a protected amino group and the chiral piperidine ring. The molecule strikes a balance: the Boc group gives stability against a range of reagents and conditions, while the overall structure is manageable and easy to manipulate in the lab. For medicinal chemists, this product streamlines multi-step syntheses by neatly sidestepping unwanted side reactions.
Customers often pay attention to melting point (ranges around 95–100°C), solubility (soluble in most organic solvents, sparingly soluble in water), and chemical purity (commonly above 97%). The Boc-protected amine stays unreactive under mildly acidic or basic conditions but sheds its Boc group in the presence of TFA (trifluoroacetic acid) or HCl, liberating the parent amine. The structure avoids extensive conjugation, so it resists photolytic breakdown. Enantiopurity matters in pharma applications, and (R)-enantiomers require careful stereospecific synthesis and verification after preparation.
Bottles arrive labeled with a chemical abstract number (CAS: 143900-44-1), molecular formula (C10H20N2O2), and batch-specific data—purity, water content, enantiomeric excess. Suppliers print storage instructions suggesting refrigeration or at least a dry, room-temperature environment, avoiding prolonged exposure to strong acids or bases outside actual use. Spectroscopic data like NMR (proton and carbon) and mass spec results help assure chemists they received a product ready for their intended application.
The usual route begins with an enantioselective reduction or resolution of a ketone or piperidine precursor. Direct Boc protection follows once the 3-aminopiperidine motif shows up, typically by treating with di-tert-butyl dicarbonate (Boc2O) under mild basic conditions—triethylamine or sodium bicarbonate tend to work. Purification depends on crystallization or column chromatography, and the chief hazards come from splashing strong chemicals or generating side-products under too much heat or haste. Manufacturers leverage automated reactors now, enabling safer, larger-batch production.
The Boc moiety tolerates a good amount of chemical abuse—that’s the main reason chemists reach for it. You might run alkylations, acylations, or even ring transformations while the Boc group guards the nitrogen. Removal is about as easy as adding it: low-temperature acidolysis delivers the unprotected amine, which then steps right into other coupling or cyclization schemes. Chemists have developed clever modifications involving inversion at C-3, cross-coupling on substituted rings, and even site-selective deprotection.
Beyond its formal name, (R)-1-Boc-3-aminopiperidine goes by several aliases such as Boc-(R)-3-aminopiperidine, 3-(N-Boc-amino)piperidine, or just “Boc-protected 3-aminopiperidine” on lab shelves. Certain catalogs even shorthand it as 143900-44-1, banking on the global language of CAS numbers. A chemist quickly learns to keep an eye out for these synonyms, since suppliers love to rebrand variations with subtle tweaks.
Most chemists notice that the solid poses little risk under normal use, but dust inhalation always stays on the radar—not many people want embedded particles in their lungs. Gloves, goggles, and well-ventilated fume hoods block most routine hazards. Chemical labels warn of potential irritation if the solid contacts skin or eyes or gets ingested. Waste disposal follows typical organic lab practice, with acid deprotection residues routed to appropriate waste bins. Overexposure to the piperidine moiety, especially in large-scale manufacture, can lead to headaches, sweating, or dizziness, so industrial protocols spell out engineering controls and personal protection.
The biggest demand springs from drug discovery and medicinal chemistry. Pharmaceutical teams lean on Boc-3-aminopiperidine to make chiral amines for lead compounds. Libraries aimed at CNS-active agents abound with piperidine cores—blockbusters like paroxetine and raloxifene spring to mind—and this building block fits right into that family. Labs also test it in polymer science: piperidines anchor specialty resins, ion-exchange media, and cross-linkers. Advanced material developers see these compounds as stepping stones to custom ligands, dyes, and even catalysts.
Academic groups keep pressing for better, greener synthesis and more reliable chiral control. Automated reactors and flow chemistry have started squeezing more product out of cheaper starting materials, wasting less solvent and energy. Process chemists spend hours optimizing hydrogenation or asymmetric catalysis steps to hit tighter purity and yield targets. The molecule also shows up in studies on metabolic stability and oral bioavailability, since many piperidines push the limits of what the body will tolerate and process.
Most reports suggest minimal acute toxicity at the doses used in preclinical settings, though the molecule hasn’t been through the kind of exhaustive study that the final drug candidates must pass. Chronic effects remain poorly mapped, so teams handle test solutions with restraint and design disposal steps to avoid environmental buildup. The piperidine ring itself carries some risk of CNS effects or sensitization, prompting extra scrutiny whenever people scale up or move into production settings. Industry experts continue to push for more robust profiling before chemists see broader commercial use.
The biological significance of chiral piperidines likely keeps this compound in demand. Synthesis and purification methods look set to become more efficient as automation and greener reagents keep spreading. There’s also potential for stronger regulatory requirements, pushing suppliers to provide fuller safety data and more details about environmental fate. I expect new analogs to break into bioconjugation, custom ligand design for catalysis, and maybe later, as frameworks for emerging therapeutics. Product lines based on (R)-1-tert-butyloxycarbonyl-3-aminopiperidine grow with every patent cycle, powering a cycle of both discovery and measured caution.
Walk into any lab that works with (R)-1-Tert-Butyloxycarbonyl-3-aminopiperidine, and you’ll probably notice chemists go straight for the purity section on the product’s certificate of analysis. High-level purity matters, not just for bragging rights. Most commercially available lots list values around 98%—sometimes you’ll find 99% purity printed on the data sheet, but it’s not always identical from batch to batch. That number isn’t just a label; the difference between 97% and 99% can make or break a synthetic route, especially downstream. I’ve been on research teams where a single misjudged decimal on a reagent’s purity led to failed syntheses and hours lost hunting down contaminants.
Labs buy this amino piperidine derivative as a sort of building block for drug candidates, peptides, and all kinds of specialized molecules. Any impurity—maybe a stereoisomer, maybe a leftover solvent, sometimes oxidized gunk—is a real headache. Imagine running a tight reaction, only to find out those sneaky impurities crept in, throwing off yields or making purification tougher. Production teams can flag tiny traces using NMR or HPLC, but even those tests have blind spots.
Recently, a colleague shared an anecdote: a project to make a novel CNS-active molecule kept giving strange chromatograms. The culprit turned out to be leftover synthetic reagent embedded in the 'pure' (R)-1-Tert-Butyloxycarbonyl-3-aminopiperidine. They expected 99%, got 95%, and the remaining 5% changed the entire game. Purity shapes everything around reproducibility and safety—no scientist wants a surprise lurking in the flask.
Not all vendors are equal. Some offer extensive breakdowns with accompanying spectra, others promise high numbers but skimp on real transparency. The smart move is to rely on trusted suppliers, look for a full suite of analytical data (NMR, HPLC, GC, sometimes mass spec), and, if possible, test an aliquot independently. Peptide shops and medicinal chemistry groups often budget for this, knowing full well that cutting corners can delay projects and damage trust.
One approach I’ve found effective is keeping an archive of spectra from past orders. Comparing new material against the old can catch subtle shifts that escape routine checks. Sometimes, if things seem off, having other eyes in the lab review the readouts has saved projects from costly rabbit holes.
Some sectors push for tighter specs, pushing material above 99.5% purity, especially in pharma, where every trace impurity can bring questions from the regulatory side. It’s tempting to settle for what’s on the bottle, but real science thrives on skepticism. If labs demand more frequent, rigorous lot testing, that pressure trickles back up the supply chain—encouraging companies to refine processes and keep contaminants in check.
Given the reliance on (R)-1-Tert-Butyloxycarbonyl-3-aminopiperidine across advanced synthesis fields, transparency and verification mean as much as the label’s number. High purity protects downstream chemistry, saves effort, and upholds safety. Laboratories that prioritize detailed analysis, open vendor communication, and regular data review usually stay ahead of the worst headaches—while pushing the industry gradually to offer cleaner, better-characterized material for everyone’s bench.
Working with (R)-1-Tert-Butyloxycarbonyl-3-Aminopiperidine, or “Boc-3-aminopiperidine” for short, brings its share of storage headaches. In any research lab, the way you handle chemicals shapes the skyline of your success—or disaster. I’ve seen more than one project delay because a colleague forgot how fussy certain compounds can be, or decided that “cool and dry” could mean a windowsill above a radiator. One bad call, and what should have been a sharp white powder turns sticky, yellow, or, worst of all, useless.
This compound doesn’t tolerate heat or moisture. Those aren’t just fine-print precautions; heat speeds up decomposition. Moisture triggers hydrolysis on the Boc protective group, and once that’s gone, the compound shifts from help to hazard. The best spot for a bottle of Boc-3-aminopiperidine sits inside a tightly-sealed container, away from room air, and never next to any source of steam or solvents. I keep mine tucked in a desiccator, well labeled, beneath other shelf-stable chemicals. I recommend keeping the temperature no higher than 8°C, which points to the fridge instead of a regular shelf.
From my experience, a dry box hits that sweet spot—no fussing about ambient humidity, no surprise condensation from fridge doors left open. Silica gel packs or molecular sieves only cost a bit extra but save a ton of money in spoiled reagents. Once, during a hot summer at a previous job, a single missed day of air conditioning left several jars of chemicals sticky and brown, not just this one. Room temperature leaves too much wiggle room for disaster, especially in regions without climate control.
Organization matters more than people admit. If you’ve ever dug for a bottle in a low-lit fridge, you’ll know labels fade fast—ink beads up or vanishes with cold and moisture. I stick to thick marker labels topped with clear tape, marking purchase dates and my own short shelf-life estimates. The minute I see clumps or color change, the jar goes out. You can’t recover a dodgy batch in medicine or materials chemistry, no matter how skilled you are. In my own rotation, smaller aliquots mean less repeated opening. One big bottle lasts ages, but opening it over and over means the reactivity creeps up through cumulative exposure to air and water.
Running a research group anywhere on a grant means balancing budgets, and wasted chemicals cut deep. Boc-3-aminopiperidine isn’t the cheapest thing on the catalog. Flubbing the storage means not only running short on reagents, but budget pain when ordering replacements. Realistically, a small up-front investment in a reliable mini fridge, good containers, and weather-appropriate desiccants saves pain down the road. I keep my logbook with a simple chemical checklist, and that includes routine checks—maybe overkill, but the moment a project depends on backup, I breathe easier knowing my stock is still reliable.
There’s nothing glamorous about careful chemical storage, but it’s the backbone of results you can trust. A regular fridge audit, clear labeling habits, and airtight storage do half the heavy lifting. If group leaders encourage a culture of treating reagents like ingredients for a complicated recipe—not leftovers—that message sticks. Reliable storage builds smoother research, and fewer headaches when you’re counting on Boc-3-aminopiperidine to pull its weight.
Anyone buying supplements, CBD oil, or food ingredients runs into this question. People want a Certificate of Analysis (COA). It’s more than a piece of paper. It’s the only real way to know what’s in the bottle, bag, or jar. Trust doesn’t stretch as far as it once did. Most of us live with news stories about product recalls, tainted supplies, knockoff brands, and missing ingredients. I’ve seen enough misleading claims on packaging to last a lifetime.
Labs test products for things you can’t see or taste—heavy metals, pesticides, microbes, and the actual amount of good stuff inside. Without a COA, you’re buying blind. Real-world example: I once tried a vitamin supplement that claimed to deliver 500mg per serving. Without lab confirmation, there’s no telling if I got 250mg, 600mg, or nothing meaningful at all. It’s the same story with protein powders, herbs, even pet foods.
The numbers on federal recalls tell their own story. In 2023, contaminated food and supplement cases led to dozens of hospitalizations and even deaths in the U.S. Alone. Independent lab results might have stopped a few of those disasters early. Consumers would probably never have heard about the problem if not for the right paperwork in place.
I once worked with a small business importing bulk spices. We ordered from overseas. Shipments arrived on pallets—sealed, labeled, everything looked fine. But without the COA, we had no proof that these bags contained only turmeric and not traces of lead or other junk. Our customers expected us to check, not just trust the word of a middleman. We even lost a big grocery store contract one year because that documentation failed to materialize in time.
That lesson stings. We relied on suppliers’ promises and paid the price when quality doubts surfaced. No grocery chain was willing to stick their neck out without independent verification. If the paperwork’s missing, the whole batch stays off the shelves. No sale means big losses and a reputation you can’t easily repair.
Anyone selling a product should be prepared to offer the COA before a purchase. No “we’ll send it after you buy.” No “trust us, we test.” A good COA comes from a third-party lab, includes recent results, and covers each lot or batch number. Don’t accept a generic sheet from last year. Real transparency means matching paperwork to the product in hand. If you run a business, this question has to be routine—every transaction, every shipment.
People care about what they put into their bodies, on their skin, and in their pets’ bowls. Without proof, trust collapses. Suppliers start losing customers. Retailers lose shelf space. The answer is simple: keep up-to-date lab reports on file and share them freely. If you’re buying, demand the paperwork. If you’re selling, make it standard practice.
Chasing the lowest price won’t protect anyone from risk. Modern customers want more than a handshake deal and a glossy label. Anyone seriously competing in today’s market makes a certificate of analysis readily available, not just as a legal shield but as a sign of respect for the people who buy their products.
In the maze of chemical building blocks, (R)-1-Tert-Butyloxycarbonyl-3-Aminopiperidine sticks out for a simple reason—its protective quality in making new molecules. It’s not a compound you’ll find under your sink or in your pain relief pills, but if you care about modern medicine and innovation, its story matters.
(R)-1-Tert-Butyloxycarbonyl-3-Aminopiperidine plays a vital part in the drug research world. Developing a new drug, especially something like an antidepressant or an antiviral, involves a marathon of small, careful steps. One wrong move, and the process falls apart, costing time and a lot of money. Pharmaceutical chemists use this piperidine derivative because the tert-butyloxycarbonyl (Boc) group shields the amine during tough reactions. This simple protection gives researchers the room to build the rest of a molecule the way they need without damaging the core parts. After the main molecular work is done, the Boc group lifts off easily, letting the amine do its job in the final product.
Makers of peptide drugs rely on (R)-1-Tert-Butyloxycarbonyl-3-Aminopiperidine when building chain-like molecules for treating diseases such as diabetes or cancer. The Boc-protected amine doesn’t react with the wrong partners during synthesis. I’ve seen bench chemists use it to prevent headaches in peptide assembly, saving days of cleanup. It makes complex stacking of amino acids possible by taking wildcards out of the process. This isn’t just convenience—it cuts costs, saves chemicals, and keeps research projects moving forward.
The pharmaceutical field races constantly to make the next blockbuster molecule. Editing the shape or reactivity of drugs sometimes depends on using ingredients like (R)-1-Tert-Butyloxycarbonyl-3-Aminopiperidine. Its chiral or “handed” structure suits some tricky projects where only one specific version of a molecule can treat a disease safely. Many drugs must show the right three-dimensional structure for the body to accept or activate them—they’re picky like that. This compound lets scientists slot a single version into a growing molecular puzzle, helping them get the balance right with side effects, activity, and cost to produce.
The reach of this molecule goes outside big pharma. Academic labs working on neurological, immunological, or infectious disease breakthroughs build new chemical probes from Boc-protected amines like this one. If your research team wants to find out how a brain receptor works or track a virus’s movement through cells, you use small molecules as tools. The kind of stability and flexibility you get with this building block makes creative science possible. Its usage points to the bigger lesson: progress in science often comes from making hard things a little easier or safer.
There are real concerns—price, supply, and environmental waste. Small, specialty chemicals like this can bottleneck when factories in the supply chain hit a snag. Chemists and industry watchdogs push for cleaner, more reliable ways to make protective groups so they aren’t a sticking point in drug discovery. Investment in faster synthesis and recycling methods, maybe even bio-based alternatives, will keep the gears turning for researchers. If cleaner routes gain traction, everyone from the local research lab to the drug manufacturers stands to benefit.
Every time a scientist steps into a lab, knowing the molecular weight and chemical formula shapes every action. Take aspirin, for example; its formula is C9H8O4. The molecular weight clocks in at about 180 grams per mole. Forget the numbers and chaos follows: too much or too little in a reaction, skewed results, wasted resources. I’ve faced experiments thrown off by simple errors in calculating molecular weights. You never forget the mess of trying to clean glassware splattered with the wrong mixture.
Let’s step outside the classroom and lab. Imagine a pharmacist preparing medication or a factory running chemical syntheses. If the math on the formula goes sideways, people can get sick, factories lose cash, and entire projects run aground. In food chemistry, misreading a chemical formula sometimes means a product gets scrapped, costing thousands. I’ve watched entire shipments returned because some detail in the formula didn’t match up with regulatory expectations.
Take glucose: C6H12O6. Its molecular weight — about 180 g/mol — comes straight from the periodic table. Each element doesn’t just add mass, it determines flavor, reactivity, behavior, and stability. Back in college, I worked on a project checking the purity of commercial sugars. If the numbers didn’t add up, there was no hiding from it; contamination or mislabeling became obvious. The molecular weight told us if something sneaky was in the jar. No shortcuts, just hard math and solid data.
People often get tangled up converting between moles and grams. Multiply the number of moles by the molecular weight — that’s the move. Miss a decimal point and the whole reaction can flop. In my early days, a recalculation saved a day’s worth of effort. Double-checking facts takes minutes, but bailing out a failed batch can eat up hours.
Another tripwire: mixing up empirical and molecular formulas. One shows the simplest ratio, the other spells out the total count. Using the wrong formula knocks experiments off target. I’ve learned to write out formulas in full on lab notes and double-check the calculations before heading near the scale.
Military and aerospace sectors often demand endless documentation over chemical formulas and weights. Quality control becomes a chain of small audits. Inspectors sometimes grill workers over the basics — and for good reason. Too much risk sits in letting details slide. As a consultant, I’ve helped companies clean up after regulators flagged simple math errors. The cost stings, but the lesson sticks.
Companies tighten up with digital vaults of chemical data, reducing room for human error. Apps cross-reference formulas instantly, turning confusing old texts into clear action steps. Still, the sharpest tool is an informed eye and a stubborn habit for double-checking. Whisper-thin margins separate the right outcome from an expensive mistake.
The real-world trick is to stay alert, double-check the numbers, and treat formulas as more than a jargon list. For anyone handling chemical compounds, those numbers tell the story of success or failure. Ignore them, and pretty soon, the reminder hits. Sometimes, all it takes is one weight, one formula, and the next step falls into place.
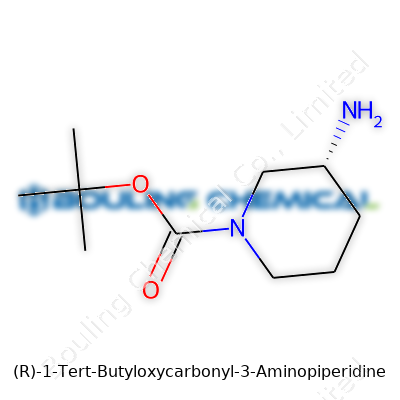
| Names | |
| Preferred IUPAC name | (3R)-1-(tert-butoxycarbonyl)piperidin-3-amine |
| Other names |
Boc-3-aminopiperidine (R)-3-Aminopiperidine N-Boc (R)-1-Boc-3-Aminopiperidine (R)-tert-Butyl 3-aminopiperidine-1-carboxylate |
| Pronunciation | /ɑːr wʌn tɜrt ˌbjuːtɪlˌɒksɪˈkɑːbənɪl θri əˈmiːn pɪˈpɛrɪdiːn/ |
| Identifiers | |
| CAS Number | 143900-44-1 |
| 3D model (JSmol) | ``` CC(C)(C)OC(=O)N[C@@H]1CNCCC1 ``` |
| Beilstein Reference | 676749 |
| ChEBI | CHEBI:131270 |
| ChEMBL | CHEMBL502054 |
| ChemSpider | 21869819 |
| DrugBank | DB08901 |
| ECHA InfoCard | ECHA InfoCard: 1007568 |
| EC Number | 87120-72-7 |
| Gmelin Reference | 113437-56-8 |
| KEGG | C21402 |
| MeSH | D010035 |
| PubChem CID | 16091842 |
| RTECS number | UF6174100 |
| UNII | 6J29W80M94 |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID5022116 |
| Properties | |
| Chemical formula | C10H20N2O2 |
| Molar mass | 215.30 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.05 g/cm3 |
| Solubility in water | Insoluble in water |
| log P | 0.93 |
| Acidity (pKa) | 10.8 |
| Basicity (pKb) | 3.32 |
| Magnetic susceptibility (χ) | -63.43 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.502 |
| Dipole moment | 4.07 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 378.7 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | No ATC code |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | `CC(C)(C)OC(=O)N1CCCC(N)C1` |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362+P364, P501 |
| Flash point | > 110.9±22.7 °C |
| PEL (Permissible) | Not established |
| REL (Recommended) | 1g, 5g |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
1-Tert-Butyloxycarbonyl-3-Aminopiperidine (S)-1-Tert-Butyloxycarbonyl-3-Aminopiperidine 1-Boc-piperidin-3-amine Piperidin-3-amine tert-Butyl 3-aminopiperidine-1-carboxylate |