Pyrrolidine doesn’t show up in everyday conversation, yet its roots stretch far back. Chemists in the early 1800s first stumbled on compounds that would later lead to the isolation of this nitrogen-containing ring. Over the decades, the hunt for easier extraction and better understanding brought scientists from Germany to England into the fold. Over time, labs started to refine its isolation, but it took the momentum of 20th-century industrial chemistry to truly kickstart mass production. I’ve seen how this chemical made a slow but steady crawl from the back pages of academic journals to the shelves of chemical suppliers. Pyrrolidine started as a curiosity and wound up an industry workhorse.
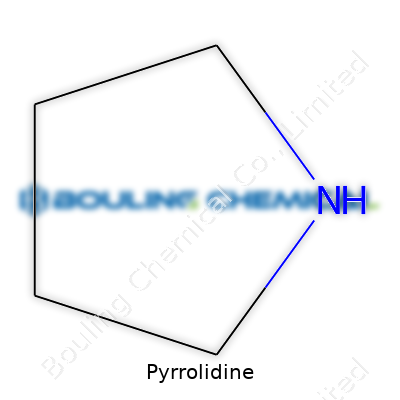
Pyrrolidine is a five-membered ring containing four carbon atoms and one nitrogen atom. Sounds simple, yet this tiny loop packs a punch in so many sectors: pharmaceuticals, pesticides, polymers, and organic syntheses all draw on its handy reactivity. I’ve seen it listed countless times in lab procurement orders, always flagged for its efficiency in driving reactions forward. In bottles, it arrives as a clear, colorless liquid. The smell hits you fast—sharp and amine-like, a reminder of its place among organic bases.
A quick look at the details: boiling point just above 87 degrees Celsius, low viscosity, and reasonable solubility both in water and in many common organic solvents. Mix it with hydrochloric acid and you’ll end up with a crystalline salt. It’s flammable, so open flames and sparks spell trouble. Like many ring-based amine compounds, pyrrolidine picks up protons easily, making it both a building block and a potential irritant. The distinct odor means spills rarely go unnoticed, even in the busiest labs. Density sits just shy of water's, and in my experience, its volatility means keeping lids tightly closed is a must.
Suppliers love to standardize: you’ll see purity listed, typically 98% or more for research-grade product. Safety data sheets warn about acute toxicity and the need for good ventilation during use. Labels mark out hazard symbols clearly, and the UN number for transport is hard to miss. Chemical Abstracts Service gives it number 123-75-1. What stands out is the insistence on gloves, goggles, and lab coats for even the smallest scale work, a reminder that familiarity doesn’t equal safety.
Industrial scale methods usually focus on cyclization of 1,4-diaminobutane or the reduction of pyrrolidone. Simple in theory, but in practice, control over moisture and purity of starting materials tips the balance between high yield and sloppy by-products. At the bench scale, I’ve watched colleagues favor catalytic hydrogenation. That need for precise conditions always gets talked about in the break room—no one enjoys a runaway exothermic reaction.
Pyrrolidine serves as a nucleophile in an array of condensation reactions. In the lab, it’s usually a base for alkylation processes and enamine formation. Reacting with acids creates pyrrolidinium salts, while treating with aldehydes or ketones triggers ring-opening or ring-changing reactions. Indoles and other heterocyclic systems grow much faster in its presence, and so many pharmaceuticals build on its ring as a scaffold. Synthetic chemists prize its ability to tweak bioactive molecules efficiently, making it a first-choice reagent for modifications.
It pops up in paperwork and lab catalogs as tetrahydropyrrole, Azacyclopentane, or just "pyrrolidine base." Occasionally, industry refers to it by trade names, but the structural name sticks, especially among research chemists. Regulatory records nearly always trail it back to the same core identity, regardless of commercial branding.
Anyone who’s spent time around pyrrolidine learns the protocols early. Flammable liquid cabinets store it away from strong oxidizers. Fume hoods are required for weighing or dispensing. MSDS sheets stress splash goggles and nitrile gloves—skin exposure triggers irritation fast, and inhalation brings headaches or dizziness. In bigger factories, sensors track any airborne traces, cutting off supply automatically if leaks spike. Disposal rules stay strict: controlled incineration, never down the sink or drain. Emergency showers and eyewash stations stay within arm’s reach whenever a bottle is open.
Pyrrolidine’s real value emerges in process chemistry. Drug companies lean on it to churn out key intermediates for anticonvulsants and cancer therapies. Crop protection products depend on its ring structure for potency. It also slides neatly into the electronics sector—cleaner manufacturing and thin-film synthesis leave no better choice for base-catalyzed steps. The plastics industry looks at pyrrolidine for making special-purpose resins and polymers. I’ve watched research teams use its flexibility to drive innovation—it’s that little backbone a molecule needs to become something bigger and more useful.
I’ve seen countless university and industry projects spin off from work with pyrrolidine. Custom derivatives feed into the search for better drugs, more sustainable agrochemicals, or efficient new materials. Researchers push boundaries by attaching all sorts of side chains and studying how biological activities shift. Machine learning and computational chemistry now predict tweaks long before any glassware is touched, opening the door to smarter, more targeted synthesis. The bulk of patent activity focuses on fine-tuning the ring’s chemical reactivity—there’s strong incentive to chase the next breakthrough by modifying a well-known scaffold.
Despite its wide use, toxicity keeps showing up as an issue. Lab rats exposed to high concentrations develop central nervous system symptoms. Skin contact burns or sensitizes fairly quickly. Long-term data remains limited, but caution dominates. Chemists log detailed incident reports, and safety committees run regular reviews. Environmental agencies want to keep it away from water supplies, noting its persistence and the risk to aquatic life. Regulations have tightened, especially where exposure could escape controlled settings.
Looking ahead, pyrrolidine's utility isn’t fading—if anything, next-generation drugs and advanced polymers only boost its importance. Pushes for green chemistry encourage recycling spent pyrrolidine and swapping out hazardous production methods. New catalytic systems could shrink energy costs and cut down on waste. Digital synthesis planning continues to open doors to more complex derivatives, making it a linchpin in the race for smarter, safer, and more effective specialty chemicals. Environmental safety drives innovation, and so do tight regulatory standards. Pyrrolidine will keep weaving through the fabric of modern chemistry, adapting to new challenges and opportunities with the same resilience it's shown for over a century.
Think of pyrrolidine as a small, lively ring-shaped building block made by chemists. Its structure looks simple—a five-membered ring, with one nitrogen atom. But don’t let the shape fool you. Pyrrolidine’s influence reaches into medicine, agriculture, and beyond.
Most of what makes pyrrolidine useful sits in its readiness to form bonds with other pieces. Unlike many larger or more complicated chemicals, kinds like pyrrolidine react quickly. In the lab, chemists use it often to make other molecules by tweaking its basic structure. Making pharmaceuticals, for example, relies on adding small changes to these sorts of rings.
I remember a college internship in a medicinal chemistry lab; every week, someone would mention pyrrolidine as a scaffold for tweaking drug candidates. It’s a little like having a base pizza crust. You can slap on different ingredients to make something new—a pain reliever, antidepressant, or maybe a promising antiviral. Pyrrolidine’s size and chemistry seem boring up close, but those features let researchers build molecules that fit into the body’s many enzyme pockets.
A handful of familiar drugs carry a pyrrolidine ring. Take nicotine, for instance—found in tobacco. Nicotine’s stimulating punch for the brain ties back to its pyrrolidine core. Pharmaceutical companies don’t stop at natural examples. They use this ring to design synthetic drugs treating a range of conditions. Some modern antiviral and anti-inflammatory medicines come out of pyrrolidine chemistry.
It doesn’t stop with medicine, either. In agriculture, scientists use pyrrolidine-based compounds to craft pesticides and plant growth regulators. By tweaking the ring, it’s possible to make molecules that protect crops or change how plants grow. These tools can help maintain crop yields, push forward food security, and nudge productivity upward.
No commentary on pyrrolidine would feel complete without mentioning safety. This chemical is nothing to play around with. Used by itself, pyrrolidine comes with a foul odor and, if handled without care, it burns the skin or irritates the airways. Like many lab chemicals, using it outside careful controls causes trouble. Industrial sites monitor air exposure, and chemists keep their gloves on.
There’s another twist. Pyrrolidine’s chemistry offers opportunities for good—but it opens the door for less savory uses. Illicit drug makers sometimes rely on this ring to assemble synthetic stimulants. Law enforcement keeps an eye on large-scale purchases, making sure bulk pyrrolidine ends up in legitimate research or manufacturing—not clandestine labs.
Getting the most out of pyrrolidine means not just admiring its versatility, but also respecting its hazards. Labs that use it store it securely, and professionals keep tight records to track where it goes. Training helps new chemists understand its quirks, so mistakes don’t happen. On the regulatory side, agencies update controls as needed to keep up with new uses. Industry can keep improving waste handling to avoid leaks into water or soil.
Pyrrolidine has made medicine more effective, crops more plentiful, and science a bit more creative. Still, making sure it serves us safely should always stay a step ahead of its risks.
Anyone who has done a bit of organic chemistry lab work knows the unique smell that lingers after using Pyrrolidine. Some call it "fishy," others say "sharp." Behind that signature scent hides a small molecule with a big job.
The chemical formula of Pyrrolidine is C4H9N. You’ve got a simple five-membered ring with one nitrogen atom. If you squint at a molecular model, it looks a little like a pentagon with a single nitrogen popping in among the carbons. That’s it—a tight, useful package. In college, as we tried to scribble out complex reaction mechanisms, I always appreciated how easy Pyrrolidine’s structure made it to sketch out.
This molecule finds its way into pharmaceutical workbenches and industrial vats more often than it gets discussed in headlines. It's a transition star in organic synthesis, especially for people wrestling with the task of building complicated rings that show up in drug molecules. Pyrrolidine’s ring structure gives chemists flexibility. Put it into a reaction, and the nitrogen atom often opens up possibilities for new bonds—good news for folks looking to tweak a drug’s properties or improve how it gets absorbed in the body.
Some days in the lab, there’s a stack of unremarkable glass bottles. The Pyrrolidine label tends to get a nod, not fanfare, simply because it pulls its weight in the background. Its job: help put together things like proline (an amino acid essential for life) or act as a catalyst in reactions when speed matters or waste needs to be minimized.
Even a modest molecule can create complications. With Pyrrolidine, safe handling is a big concern. Its vapors irritate the eyes and lungs, so people in labs lean on fume hoods and good gloves. It’s also flammable, calling for mindful storage far from sparks or open flames. Years ago, a leaky container in our student storeroom proved how quickly its odor can take over a room. That sharp lesson stuck.
On a broad scale, Pyrrolidine shares an issue with a long list of synthetic chemicals: trace amounts can escape factory walls and slip into the water supply. Studies in environmental chemistry reveal that nitrogen-containing rings don’t always break down easily. Once out in the wild, these remnants might slow down natural bacterial processes in rivers—sometimes enough to hurt local ecosystems. Good chemical stewardship matters, and companies face the ongoing challenge to boost their waste treatment and limit what gets away.
Plenty of industries benefit from tiny workhorses like Pyrrolidine, but broader debate grows louder about chemical sustainability. For people working with molecules, there’s pressure to think about safer alternatives, greener solvents, and reactions that generate less byproduct. In practice, a shift toward recycling and “closed-loop” chemical systems could help limit leakage. Ongoing research focuses on new catalysts and ring systems that mimic Pyrrolidine’s usefulness without the same footprint.
Some days, breakthroughs feel slow. Yet daily effort—everything from careful storage to waste reduction—keeps chemistry’s hidden helpers from causing more harm than good. Pyrrolidine’s value doesn’t just come from its formula, but from the responsibility to handle it wisely in labs, factories, and beyond.
Few people outside the chemistry crowd recognize pyrrolidine, but for those who deal with organic synthesis, its name rings a loud warning bell. Pyrrolidine looks as harmless as water, and it has no flashy color or attention-grabbing odor. But dig a bit deeper, and you see a different story—one driven by hard science, lived experience, and some nasty surprises.
I still remember my first exposure in an undergraduate chemistry lab. The instructor made a point to highlight certain bottles with red tape, and pyrrolidine belonged in that group. Sure, the label listed “flammable” and “corrosive,” but the true hazard came clear only after reading the material safety data sheet. People get careless with chemicals that look tamer than sulfuric acid, and accidents happen that way.
Pyrrolidine evaporates quickly, and the fumes irritate the nose and throat almost instantly. Shortness of breath and headaches tend to follow when ventilation falls short. Contact brings a different set of problems—skin starts to itch, turn red, then blister. Eyes sting and water, and vision blurs unless the wash station is nearby and you use it fast. Long sleeves and goggles suddenly seem less like overkill and more like basic self-preservation.
Toxicity isn’t limited to short-term discomfort. Studies from the National Library of Medicine report that repeated exposure kills cells, damages internal organs, and could raise the risk of some cancers over time. Rats exposed to high levels suffered liver damage, a worrying thought for anyone spending long hours near an open container. The lower the exposure, the better off you end up.
Beyond the physical problems, pyrrolidine has a tendency to catch fire. It burns with a nearly invisible flame, throwing off sparks and smoke only once the fire grabs something in its path. Mixing it with certain acids or oxidizers sets off violent reactions. I’ve seen a benchtop go up in seconds because someone failed to recognize a residue from a previous experiment.
It’s easy to take shortcuts with common lab solvents, but pyrrolidine demands respect. Leaving lids unscrewed, skipping gloves, or pouring leftovers down the sink are choices that put everyone in danger. Those shortcuts cost time, money, and sometimes health. I once worked with a technician who developed breathing difficulties after careless handling over a few months. Simple accidents, like splashes or inhaling too much vapor, can end careers.
Most problems with hazardous substances boil down to habits and equipment. Fume hoods matter more than open windows. Proper gloves and goggles mark the difference between a close call and a trip to urgent care. Labels, checklists, and training aren’t paperwork—they keep people safe. I once watched students treat chemical safety like an afterthought—until a single spill cut the class size in half for weeks.
People in industrial settings already keep pyrrolidine behind multiple barriers: locked cabinets, restricted access, monitored air quality. Small labs and classrooms lag behind, but even simple steps lower the risk. Read the data sheet, ask for better ventilation, and call out unsafe practices. Sharing stories about close calls does more to change behavior than any policy ever will.
Chemistry never comes without risk. Treating pyrrolidine like just another lab supply pushes those risks higher. Respect for the damage it can do protects the people who work with it and keeps dangerous mistakes from making the news.
Anyone who’s spent time in a lab or chemical store will tell you: pyrrolidine doesn’t just quietly do its job. It has a sharp smell, tends to leak if you slack and reacts quickly to air and moisture. Leave a bottle open or let it get too warm and you’re asking for more than just a strong whiff. Like other volatile amines, pyrrolidine gets out of hand fast—vapor escapes, cap gaskets give out, and sometimes the container itself softens or stains. That stuff stains fingers, benches and leaves stubborn odors for days. I remember learning this the hard way during my early research days, walking in and finding the whole room reeking because a flask lid hadn’t been tightened.
Shelving decisions aren’t just about convenience. Sitting pyrrolidine next to acids or oxidizing agents is like keeping matches and gasoline side-by-side. One spill, one splash, and suddenly you aren’t dealing with a routine clean-up, but a scary situation with nasty fumes or, worse, a fire. The best shelf for this chemical sits away from heat or sunlight, up high, outside the humidity range of most labs, far from corrosives. Metal cans corrode and stained old jugs sometimes fail utterly, especially after months of exposure to fumes. Choosing thick, airtight glass bottles with PTFE-lined caps has saved me a lot of headaches.
People think labels are busywork until something leaks and nobody knows what’s inside. One late night, someone thought they’d found a solvent for routine cleaning and instead nearly passed out from the vapors. Every bottle of pyrrolidine should wear bold, chemical-resistant labels. Not just the name, but the hazards: “Flammable,” “Corrosive,” “Causes Severe Burns.” Peeling, faded stickers become as useless as ignoring a stop sign.
Pyrrolidine vapors burn the skin and eyes, sometimes before you even know there’s a leak. The burns are sneaky—tiny droplets splash further than you expect, clinging to gloves and door handles. I now always store it in a dedicated secondary containment tray, high-walled, that can catch drips or spills. Chemical storage rooms run on redundant safety—so every time I hear about a new accident, I wonder whether someone skipped the tray or left the cap loose. For even short-term storage, I keep materials close: nitrile gloves (not latex—those dissolve!), full goggles, thick aprons, fume hoods nearby.
Heat is the real enemy. Pyrrolidine boils at not much more than water at sea level. Warm storage pumps up vapor pressure, driving more chemical into the air even through an apparently tight cap. Once, a container left near a sunny window swelled and cracked, stinking up the whole hallway. Now, I keep it where the room feels chilly, and double up by storing it in a flame-proof cabinet if the weather runs hot. The threat isn’t just spoiling the chemical – it’s personal safety, building safety and, honestly, peace of mind.
Good storage doesn’t need fancy tech: just vigilance, clear labeling, real physical barriers, and strict temperature control. Rotate stocks, toss containers that show the least sign of swelling or corrosion, and always check that cap twice before you step away. A few minutes extra care spares everyone from unnecessary scrapes, burns, ruined experiments and ruined days.
Walk into any pharma, agrochemical, or specialty chemical plant and odds are good that pyrrolidine makes an appearance on the ingredient list. In the world of organic chemistry, this five-membered ring does a lot of heavy lifting. For most people, it stays out of sight, and that's a shame, because this little molecule plays a bigger role than it gets credit for.
Drug development has always relied on tools that add complexity to simple structures. Pyrrolidine steps up as both a foundation and a supporting character. Many medicines, from anti-infectives to drugs for heart disorders, build on this framework. Take the case of proline, one of the original amino acids–the core is pretty much a pyrrolidine. Turn to the shelves full of antihypertensive drugs and you will spot structures where it pops up again and again. Production of new molecules isn’t easy. Speed, purity, and stability matter, and pyrrolidine-based scaffolds help keep chemists in control throughout synthesis. By providing a tested structure, pyrrolidine shortens development time and gets treatments into patients’ hands faster. Anyone who has worked in pharmaceutical R&D knows that a reliable intermediate like this can make projects less nerve-wracking.
Out in the fields, crops face attacks from all directions. To defend them, scientists build protection chemicals that can take a beating—fungicides, herbicides, and insecticides. Syringing a boost of resilience or improving uptake depends on solid chemical design. Pyrrolidine shines here by giving molecules the toughness and water compatibility needed for the field. Take the example of key herbicides; where stability makes or breaks crop yield, pyrrolidine structures hold up well under rough conditions. In regions where constant pests could mean ruin for a year’s work, this is not just important—it keeps food available.
Many people in industry, myself included, have seen how key reactions stall out without the right solvent or catalyst. Pyrrolidine acts as both. For chemists pushing reactions that seem stubborn or messy, this means smoother operations and better yields. Its use as a solvent in round-the-clock production lines speaks to its effectiveness and straightforward handling. Much of the specialty coatings and plastics industries depend on such reactions to deliver products that hold up to scrutiny.
Ever handled paint that just seemed smoother or a finish that stayed bright for years? Material chemists credit molecules like pyrrolidine for that kind of longevity. Its chemical structure means it holds onto desired traits while resisting unwanted reactions. This property comes in handy for adhesives, sealants, and resins. Electronics manufacturing also borrows some of these features to produce circuit board coatings that survive harsh environments.
Working with pyrrolidine means dealing with a compound that can be hazardous. My own days in the lab taught me respect for gloves, goggles, and proper ventilation. Not everyone takes these precautions, leading to risks that could be avoided. Companies should invest more time in training and smart controls rather than cutting corners. At the supply level, staying ahead of market demand prevents shortages—one rough harvest or plant shutdown and prices shoot up. By ramping up local production and encouraging recycling or waste minimization, manufacturers can keep pyrrolidine available and affordable.
| Names | |
| Preferred IUPAC name | pyrrolidine |
| Other names |
Azacyclopentane Tetrahydropyrrole |
| Pronunciation | /paɪˈrɒlɪˌdiːn/ |
| Identifiers | |
| CAS Number | “123-75-1” |
| Beilstein Reference | 505012 |
| ChEBI | CHEBI:35521 |
| ChEMBL | CHEMBL417 |
| ChemSpider | 5827 |
| DrugBank | DB02907 |
| ECHA InfoCard | 100.005.552 |
| EC Number | 208-876-7 |
| Gmelin Reference | 7907 |
| KEGG | C01772 |
| MeSH | D011693 |
| PubChem CID | 31248 |
| RTECS number | UY1575000 |
| UNII | 7I7VM0VDKO |
| UN number | UN2402 |
| CompTox Dashboard (EPA) | DTXSID4020715 |
| Properties | |
| Chemical formula | C4H9N |
| Molar mass | 73.12 g/mol |
| Appearance | Colorless liquid |
| Odor | amine-like |
| Density | 0.866 g/mL at 25 °C |
| Solubility in water | Miscible |
| log P | -0.83 |
| Vapor pressure | 5.1 kPa (20 °C) |
| Acidity (pKa) | 11.3 |
| Basicity (pKb) | 2.73 |
| Magnetic susceptibility (χ) | -20.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.458 |
| Viscosity | 2.0 mPa·s (20 °C) |
| Dipole moment | 1.80 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 274.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −89.7 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -3961 kJ mol⁻¹ |
| Pharmacology | |
| ATC code | N07XX08 |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS06, GHS08 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H225, H302, H314, H336 |
| Precautionary statements | H225, H302, H314 |
| NFPA 704 (fire diamond) | 2-3-0 |
| Flash point | -15 °C |
| Autoignition temperature | 340°C |
| Explosive limits | 1.8% - 14% |
| Lethal dose or concentration | LD50 oral rat 350 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 300 mg/kg |
| NIOSH | TTT7810000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 40 mg/m3 |
| IDLH (Immediate danger) | 150 ppm |
| Related compounds | |
| Related compounds |
Proline 2-Pyrrolidone Pyrrole Piperidine |