Chemists have worked for decades to push the limits of pyrrolidine derivatives. Pyrrolidine-3-ol first drew interest in the middle of the 20th century, during a time when advances in heterocyclic chemistry were opening paths to countless new compounds. Researchers discovered that tweaking the backbone of pyrrolidine produced molecules with an extraordinary range of properties. With every adjustment to the ring, scientists found new possibilities for pharmaceutical and agricultural development. Looking at the literature from the past sixty years, you will notice waves of studies, each one building on purer syntheses, more reliable analytical methods, and broader insights into how these molecules fit into the world of bioactive chemicals. Many of these efforts led directly to products people use today, whether in research labs or in more industrial settings.
Pyrrolidine-3-ol is a small, cyclic amine packed with functional potential for researchers and manufacturers. With a five-membered ring system holding a hydroxyl group neatly at the 3-position, it straddles the line between simple building block and chameleon-like starting point for more complex molecules. Chemists clip, swap, or bond this scaffold time and again because it combines stability with the kind of reactivity that opens doors to whole new chemical families. Suppliers package it as a solid or in solution, ready for practical uses in the lab or production site. Once chemists get hold of it, they can head in molecular directions limited only by their goals.
Pyrrolidine-3-ol appears as a white to off-white powder at room temperature. Somehow, the molecule balances modest water solubility and a tendency to dissolve in most polar organic solvents, giving it broad practical value. Its boiling point lands slightly above 200 °C, and it melts somewhere between 65 and 69 °C, making storage and handling manageable without too much special equipment. Smelling faintly amine-like, the compound stands up well under the standard operating conditions in research and light manufacturing. Chemically, the key sites of action are its nitrogen atom and the hydroxyl group—two points where new bonds often form in follow-on reactions. In the chemistry lab, minor differences in crystalline form, purity, or particle size rarely affect its performance, so long as standard analytical methods confirm identity and purity with NMR, HPLC, or MS.
Common suppliers offer pyrrolidine-3-ol at purities of 97% or higher, with certificates of analysis and lot numbers for traceability. Labels note molecular weight (approximately 87.12 g/mol), batch, storage instructions, and health cautions concerning skin and eye contact. The CAS number 89132-31-8 appears on every package, a necessary reference in procurement and hazard communication. Some stockists highlight the specific stereochemistry (if a certain enantiomer has been synthesized) because biological properties can change dramatically between forms.
Traditional synthesis routes start from readily available starting materials such as butyrolactone or gamma-aminobutyric acid. Chemists close the ring using a reductive amination or cyclization. Attention to pH, timing, and temperature makes all the difference in maximizing yield and purity, since side reactions lurk at every step. My own experience in the lab bears this out: small changes in conditions often mean the difference between a pile of side products and a clean solid you can count on. Many labs now employ catalyzed hydrogenation approaches to achieve cleaner conversion, and automated purification systems cut down post-reaction work.
Researchers and manufacturers value this compound for its handleability—both the nitrogen and the alcohol group serve as entry points for functional group installations. Alkylation or acylation of the nitrogen allows further tailoring, while the alcohol can be turned into a leaving group for nucleophilic substitution or oxidized to a ketone. Chemists sometimes convert it into esters or ethers for greater control over solubility or reactivity in follow-on work. In medicinal and fine chemical circles, people often use it as a "building block" to push into analog libraries or specialty intermediates.
Pyrrolidine-3-ol appears in the literature and on product sheets under several aliases, including 3-Hydroxypyrrolidine and 3-Pyrrolidinol. Other less common tags include γ-hydroxyproline and β-aminopropanol cyclic derivative, though most scientists stick to the IUPAC naming or one of the two main synonyms. Catalog numbers differ by supplier, but the CAS number stays the same for avoiding ambiguity.
Handling this compound means watching out for typical amine-related hazards: eye and skin irritation, unpleasant odor, and the need for gloves and goggles. Storage usually calls for well-ventilated, dry places away from oxidants. Material safety data sheets lay out the basics, but experienced chemists know better than to trust even simple molecules without a safety check. Pyrrolidine-based compounds have a reputation for volatility and sometimes surprise reactivity with acids or oxidants, so double-checking storage rules and keeping containers sealed goes a long way. Clear labeling and staff training hold equal importance.
The uses of pyrrolidine-3-ol run across pharma, agrochemicals, and fine chemicals. Medicinal chemists often turn to it as a scaffold for potential drug candidates, especially for neurological and anti-infective research. Industrial production trials have used pyrrolidine-3-ol as a starting point for specialty intermediates and as a component for active ingredients in pesticides and herbicides. The high reactivity of its nitrogen and hydroxyl groups unlocks pathways to compounds not easily accessed otherwise. Academic labs studying new routes to chiral molecules keep it on hand for its ability to steer stereochemistry in pivotal reactions. Many researchers tinker with derivatives to assess biochemical properties or find leads for new targets.
Chemists have explored dozens of new pyrrolidine-3-ol analogs to enhance bioactivity, solubility, or selectivity. In my own experience, this often means late nights at the bench, running NMR after NMR to confirm minor changes and tweak conditions for the next analog. R&D teams reach repeatedly for this scaffold to prototype new chemical entities, sometimes as enzyme inhibitors or as part of modular synthesis platforms for drug libraries. Partnerships between academia and industry build up databases of structure-activity relationships, and high-throughput screening pushes the field forward by rewarding both positive and negative results alike.
Toxicology testing covers basic acute exposure, metabolic breakdown, and longer-term effects. The data pool for pyrrolidine-3-ol remains relatively modest compared to larger volume chemicals, but initial evidence suggests moderate systemic toxicity at high doses and potential irritation on contact. Animal studies inform limits for lab use, and many institutions set internal limits lower than the general guidance. Anyone responsible for workplace safety keeps these records close at hand and makes sure every handler knows the warnings—not just in theory, but as part of regular workflow.
Looking ahead, pyrrolidine-3-ol and its kin seem set to remain fixtures in chemical development and process chemistry. Every year brings new papers mapping out bioactive analogs or improved synthetic routes. Advances in automated synthesis, better catalysts, and greener solvents may bring down costs and push wider use in both pharmaceuticals and materials science. Chemists will keep reaching for this molecule to form the heart of new ideas—sometimes for drugs, sometimes for chemicals no one has dreamed up yet. With ongoing discovery in both basic reactivity and applied settings, the road ahead for pyrrolidine-3-ol runs wide open, ready for anyone with a new approach and the tenacity to see it through.
Pyrrolidine-3-ol isn’t something you’d come across at a hardware store, but in a medicinal chemist’s lab, it’s as useful as duct tape. Labs use it to add that special twist to pharmaceutical molecules. Its structure can help a compound fit into the lock of a protein, triggering just the right response in the body. Several research teams have explored modifications of pyrrolidine-3-ol, hoping to create new treatments for conditions from depression to viral infections. I once watched a colleague spend weeks tweaking a similar five-membered ring—finally stumbling onto a version that lit up the screens in our lab’s bioassays. Compounds with these rings can end up in preclinical studies and sometimes make their way into medicines, especially those looking to target enzymes in the brain or immune system.
Farming has its own chemistry. Agrochemical companies look for just the right balance: strong enough to handle pests, gentle enough to not toast the crops. Pyrrolidine-3-ol derivatives sometimes slip into pesticides and herbicides after researchers find they can poke holes in insect enzymes, but leave plants unbothered. Success in this area tends to mean better yields and fewer crop losses, which matters for both farmers and the millions of people those crops will feed. I know the distaste folks sometimes have for “lab chemicals” in their food chain, but most advances in this sector have to meet safety standards set by agencies like the EPA and EFSA.
Chemists aren’t limited to medicines or farm fields; they’re always hunting for new materials, sometimes just for the thrill of what might happen. Pyrrolidine-3-ol can act as a starter piece for polymers—those flexible, sometimes stretchy plastics used for everything from phone cases to auto parts. Its alcohol group (the -ol) turns out to be a practical handle: it reacts cleanly with acids and other building blocks, stringing together long chains. Material scientists can then fine-tune these chains, aiming for elastomers that last longer or insulators that hold up to heat. With electronics shrinking, those properties become more valuable.
Grad students and research scientists rely on things like pyrrolidine-3-ol to probe the rules of chemistry and biology. Its unique shape allows for testing enzyme selectivity or making labeled molecules that help trace biochemical pathways. Radiolabeling or fluorescent tagging often starts with a molecule that has a functional handle already in place—just like the alcohol group here. My own stint in graduate school showed me how a well-placed group in a small molecule could make a synthesis miles easier, sometimes saving weeks in the lab. For those aiming to map how molecules move in living systems, these shortcuts offer real value.
Pyrrolidine-3-ol has a lot going for it, but chemical safety is not a hands-off matter. The compound and its derivatives sometimes need careful handling due to reactivity and, in some cases, toxicity. That means teams need training and the right equipment—gloves, hoods, and knowledge of what to do if something goes wrong. There’s been a bigger push in recent years for greener chemistry: finding routes that produce less waste, use fewer harsh reagents, and rely on safer solvents. Some labs have started to swap in renewable starting materials or use water-based reactions when working with alcohol-bearing rings—pointing to a future where useful molecules like this can be crafted with less impact.
Pyrrolidine-3-ol sounds like one of those names that filters through chemistry lectures without much fanfare. To many, it’s just another small organic molecule, an offshoot in the endless family tree of heterocyclic compounds. At first glance, it may not hold the cachet of nicotine or caffeine, but the nuts and bolts of its structure tell a story just as relevant in research labs and, indirectly, in real-world applications.
Diving into the chemical makeup, pyrrolidine-3-ol delivers a pyrrolidine backbone. This is a five-membered ring containing four carbon atoms and one nitrogen atom, which forms a closed loop — think of something like a miniature bracelet where nitrogen takes the place of a bead. At the third position of this ring, an -OH group (hydroxyl) jumps into action, shifting its chemical behavior significantly.
The actual molecular formula looks like this: C4H9NO. To sketch it out with a pencil, you’d number the ring atoms (with nitrogen as number 1), then tack on the hydroxyl group to carbon at spot 3. Every time I see molecules like this, I picture them not as flat diagrams but as flexible loops, twisting and bustling as they meet other molecules in a flask or inside the body.
It’s easy to breeze past small chemicals, yet small adjustments like adding a single oxygen atom can set off big changes. That -OH group means this molecule can play nicely with water, making it more likely to dissolve in a living cell or lab solution. If you work with chemistry, you know the value in swapping one functional group for another, giving rise to new reactions, properties, and even pharmaceuticals.
For instance, derivatives of pyrrolidine pop up all over drug design. Chemists start with the plain ring, add or swap groups, and watch as the biological activity changes — sometimes drastically. That added hydroxyl provides a handle for reactions, letting researchers steer the molecule toward new functions. I remember running reactions with similar compounds during my own research; sometimes, a tiny tweak like this is all it takes to unlock unexpected results.
Pyrrolidine-3-ol won’t win a place in the spotlight the way aspirin or ethanol does. Yet without these foundational chemicals, much of biomedical science loses its footing. Right now, you can track its fingerprints in pharmaceutical intermediates or in building blocks for materials. In chemical supply catalogs, it doesn’t grab headlines — but in synthetic routes where tiny rings and hydroxyl groups change the rules of engagement, it matters a lot.
Handling molecules with reactive groups always calls for respect. The hydroxyl moiety, while handy, can open the door to unwanted side reactions in large-scale processes. This adds a layer of challenge to chemical manufacturing, where costs and purity stack up quickly. Having worked in both academic and industrial settings, the headache of purification and side products is never far away. More targeted catalysts and greener solvents seem like a smart way to cut down on both waste and energy use, an area where research keeps grinding forward.
As chemists look for new scaffolds and greener reactions, molecules like pyrrolidine-3-ol will keep turning up behind the scenes. They serve as reminders that chemistry’s elegance often hides in these small, flexible rings, always open to change and full of possibility.
Take a bottle of Pyrrolidine-3-ol off the shelf, and you can smell a hint of danger right away. My first year handling chemical intermediates brought me across small vials with big risks, and nothing makes you rethink your approach quite like a close miss with a spilled solution. Storing Pyrrolidine-3-ol isn’t about finding any old spot in a cabinet. This stuff reacts if you get careless—not in big, Hollywood-explosion ways, but in slow, silent leaks and slow-burn irritations you won’t notice until it’s too late.
The bottle belongs in a cool, lockable chemical cabinet, away from heat, open flames, and sunlight. Bright light can break down organics over time, and heat accelerates that process. Even a sunbeam from an open window has been known to spoil a batch of sensitive reagents. Once, a back-corner bench near a heating pipe made a sample fizz up without warning. It’s a plain reminder: don’t let chemicals sit near forgotten heat sources. Humidity can mess with some compounds too, and Pyrrolidine-3-ol enjoys sticking its nose into moisture where it doesn’t belong, so always twist that cap on tight.
Separate your storage. Acids, bases, oxidizers, and organics all need their own spots. Mixing Pyrrolidine-3-ol with the wrong neighbor can trigger dangerous reactions. Over the years, I’ve seen the mess that results from stacking bottles of incompatible substances—sticky residues, weird smells, and sometimes an entire shelf lost to contamination. If you care about long-term health and not ruining your equipment, you make the labels bold and keep the inventory updated.
Opening a bottle feels routine, but let your guard down and you’ll pay for it. I learned this after a single splash landed on my arm and sent me hunting for the eyewash station. Lab coats, splash-proof goggles, and gloves aren’t for decoration—they’re your only line of defense. Nitrile gloves hold up well when working with Pyrrolidine-3-ol, and I’ve watched vinyl gloves dissolve with other solvents in minutes. Always give the work area a good once-over before starting and keep your eyewash and spill kits close by.
A fume hood earns its spot in every decent chemistry workspace. Pyrrolidine-3-ol doesn’t belong out in the open air—its vapors go right for mucous membranes and turn a fine afternoon into a coughing fit. Run small reactions and weigh out samples only in a ventilated enclosure. Once, I tried “just a quick transfer” outside the hood and ended up regretting it, hacking away and swearing to never cut corners again.
Disposal rules seem like a headache, but they exist for a reason. The drain isn’t a shortcut. Used Pyrrolidine-3-ol belongs in its own labeled waste container, away from acid or oxidizer waste. Contaminated gloves, pipette tips, and paper towels should never hit ordinary trash bins. One wrong move with mixed chemical waste can give you a nasty surprise months later, with containers swelling, leaking, or spraying when opened. I always check for solid guidance from material safety data sheets or a knowledgeable environmental health office before tossing or pouring anything.
Safety culture sounds like a buzzword, but one small slip can land you or your co-workers in the nurse’s office. So far, I’ve seen teams who respect each other by cleaning up immediately, labeling everything twice, and hand-delivering reminders about upcoming inventory checks. Clear language, repeated training, and a real willingness to stop and double-check, even when someone acts impatient, create an environment where Pyrrolidine-3-ol and other chemicals stay under control, not the other way around.
Ask around in any chemistry department or a material science lab and folks working with Pyrrolidine-3-ol will tell you: purity matters a lot. In lab work, even a tiny contaminant can throw off months of hard-earned data. So, labs look for high-purity Pyrrolidine-3-ol—something that clocks in above 98% by most HPLC or GC standards. This isn’t about being picky. Some projects, especially pharma or biotech, require almost surgical cleanliness in their chemicals. I’ve had colleagues whose experiments fell apart over 0.5% of the wrong impurity.
Move away from the analytical side, and the story changes. Factories running chemical syntheses at scale often pick a lower-purity form to keep costs reasonable. Nobody wants to pay for 99.9% ultra-grade chemical if 95% gets the job done without fuss. Sometimes, that 5% gap has things you wouldn’t want in a biological system—maybe unreacted precursors or common byproducts—but manufacturing folks might not worry about those the same way a pharmacist would.
I’ve seen cases where suppliers offer multiple grades—one for research, one for production, sometimes one “technical” grade that covers things like coatings or other industrial uses where precision isn’t so critical. If you work in or supply to those industries, you end up needing to juggle different batches and labels—analytical, technical, and sometimes “pharma” grade, especially if clinical work enters the equation.
Here’s the kicker: "grade" means something different wherever you stand. Labs often check for exact levels of water, heavy metals, or even unknown peaks. Industrial teams might get a spec sheet with a main assay value and very little else. That gap between expectations can lead to missed emails or, worse, a costly production halt. I once had a project where the grade didn’t match what we ordered—a delay that cost more than upgrading to the cleanest batch in the first place.
Some providers do their homework and test for everything you’d expect as a buyer: not just purity, but also stability over time, right packaging to avoid hydrolysis, and even documentation about residual solvents. Not every vendor will, though. If something as basic as a Certificate of Analysis is missing or vague, I’ve learned the hard way it’s better to push back and demand answers up front.
On paper, this sounds like a supply chain formality. In practice, gaps in purity can mean failed batches, noisy results, or in worst-case scenarios, legal trouble if a mislabeled drum ends up in a regulated product line. Pushing for better transparency—from suppliers and buyers—saves hassle in the long run. It’s tempting to grab whatever bottle shows up on a search, but a quick check on what you’re really getting will save you time, money, and maybe your job.
Calls for clearer labeling and agreed-on minimum standards make sense. Putting those into practice rarely comes easy unless everyone shares the cost. Some buyers start demanding regular third-party analysis or start working exclusively with companies that invest in quality control. Building that trust pays off, especially as Pyrrolidine-3-ol finds uses in more sensitive work. If you’re buying it, or selling it, treating every grade as a unique animal makes life easier for everyone involved.
Pyrrolidine-3-ol sounds like something out of a dusty chemistry textbook, but it shows up in the life science world more often than you’d guess. As a building block for a wide range of chemicals and pharmaceuticals, people who work in labs spend a lot of time handling it. At home, most folks never see it, but it’s worth paying attention if you’re working in research or industrial spaces. Chemicals with an "-ol" group often bring unique risks, and this one isn’t just a harmless molecule floating around without consequence.
I’ve spent enough time in labs to know people let their guard down with intermediates like Pyrrolidine-3-ol. Shelf bottles go without labels. Gloves stay off. This gets risky fast. On skin, the compound can cause redness, itching, and rashes. Accidental eye contact? Prepare for pain, irritation, and possibly long-term damage if you don’t flush it out fast. You might look at a technical sheet and see words like “moderate irritant,” but a simple splash on the skin has sent more than one researcher home with swelling or chemical burns.
Vaporized Pyrrolidine-3-ol isn’t something you want in your lungs. Fume hoods and proper masks keep most of the staff safe, but little mistakes lead to big problems—a cracked bottle, or someone pouring too quickly, and there’s headache, dizziness, and nausea. For anyone with asthma or respiratory issues, exposure can ramp up the severity. In smaller startups or university labs where money gets tight, proper ventilation sometimes slips down the budget list. The consequences pile up quickly, from minor coughs to ER trips for more severe symptoms.
Most folks read about “acute toxicity” and feel safe after a shift. Long-term exposure worries don’t get enough attention. Laboratory studies suggest repeated contact over weeks or months damages liver function and disrupts neurological health in animals. While hard human data’s in short supply, the risks shouldn’t be ignored just because the science hasn’t caught up yet. In my own experience, labs that skip regular health checks or downplay long-term warnings pay dearly later, with staff reporting memory problems and fatigue.
Chemical risks aren’t inevitable. Supervisors who actually train their teams and insist on real-time label checks put a big dent in accidents. It hardly takes five minutes each day to walk through the standard safety data sheets as a group, but those minutes save hospital visits. Even cheap disposable gloves and face shields stop most exposures. In labs I’ve run, we scheduled monthly ventilation audits and restocked personal protective equipment twice as often as the official guidelines suggested. That approach cut accident reports in half.
Companies and universities with tight budgets sometimes treat chemical safety as a box-ticking exercise. That shortcut often leads to legal costs down the road. The voice of experience says: buy one less gadget if you need to, but never skimp on equipment that keeps you protected. Regulators should also keep up pressure so safety standards don’t slip as new uses for Pyrrolidine-3-ol show up.
Anything used to make food additives or pharmaceuticals leaks into the supply chain if not handled right. Residues get on workers’ hands, then sit on doorknobs, coffee machines, or paperwork. Years ago I saw a grad student handle his lunch with unwashed hands after a synthesis run. He ended up with GI distress for days. It’s a small example, but it sticks. Safety around Pyrrolidine-3-ol doesn’t only matter for the person doing the mixing—it follows you into common spaces, which puts pressure on everyone to keep habits sharp.
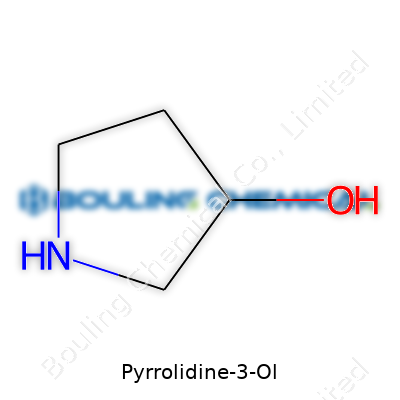
| Names | |
| Preferred IUPAC name | Pyrrolidin-3-ol |
| Other names |
3-Hydroxypyrrolidine Pyrrolidin-3-ol 3-Pyrrolidinol |
| Pronunciation | /paɪˈrɒl.ɪˌdiːn.θriː.ɒl/ |
| Identifiers | |
| CAS Number | 4972-26-7 |
| 3D model (JSmol) | `3Dmol-cid-10479536` |
| Beilstein Reference | 081114 |
| ChEBI | CHEBI:191217 |
| ChEMBL | CHEMBL2173684 |
| ChemSpider | 119380 |
| DrugBank | DB08798 |
| ECHA InfoCard | 050000016397 |
| EC Number | 215-559-4 |
| Gmelin Reference | 84922 |
| KEGG | C02901 |
| MeSH | D051518 |
| PubChem CID | 12514169 |
| RTECS number | UQ2325000 |
| UNII | PY6T2G9E5F |
| UN number | UN3276 |
| CompTox Dashboard (EPA) | DTXSID0044368 |
| Properties | |
| Chemical formula | C4H9NO |
| Molar mass | 87.122 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | Amine-like |
| Density | 0.987 g/cm3 |
| Solubility in water | miscible |
| log P | -0.54 |
| Vapor pressure | 0.4 mmHg (at 25°C) |
| Acidity (pKa) | **11.3** |
| Basicity (pKb) | 5.93 |
| Magnetic susceptibility (χ) | -52.02·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.457 |
| Viscosity | 7 mPa·s (lit.) |
| Dipole moment | 1.89 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 296.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -182.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3894.8 kJ/mol |
| Pharmacology | |
| ATC code | N07XX10 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | ⟦GHS07⟧ |
| Signal word | Danger |
| Hazard statements | H302, H314 |
| Precautionary statements | P264, P270, P273, P280, P301+P312, P305+P351+P338, P308+P311, P501 |
| NFPA 704 (fire diamond) | 1-2-0-W |
| Flash point | 73.9 °F |
| Lethal dose or concentration | LD50 (Rat, oral): >2000 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Pyrrolidine-3-Ol: 1600 mg/kg (rat, oral) |
| NIOSH | STEL 6 mg/m3 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 20~25°C |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Pyrrolidine Prolinol Proline Pyrrolidone Piperidine |