Pyrrole entered the world of chemistry down a humble path. In 1834, the chemist F. F. Runge stumbled on its distinctive smell during an experiment with coal tar, unaware he’d uncovered a building block for future chemistry. The name comes from the Greek word for fire, connecting its smoky odor to its history. Through the years, curiosity grew about this nitrogen-containing five-membered ring. By late 19th century, scientists like Bunge and Baeyer mapped out the structure, recognizing its intimate role in more complex compounds like heme and chlorophyll, both essential to life. Such discoveries put pyrrole on the map—not as a headline grabber, but as a silent partner in biological processes. For those interested in chemical history, pyrrole’s path from coal tar oddity to core bio-molecule seems like a sturdy testament to science’s knack for finding value in the overlooked.
Chemists see pyrrole’s value in its flexibility and ease of modification. It’s a small molecule, but it packs a punch. Colorless to slightly yellow in appearance, it often shows up as a volatile liquid under common lab conditions. Industrial labs order pyrrole as a reagent for synthesizing pharmaceuticals, agricultural products, and specialty polymers. The highly reactive nature means it rarely sits on a shelf for long. Its role as a precursor in the synthesis of porphyrins—a vital group that includes hemoglobin and chlorophyll—is critical, and many industries tap its benefits for both research and industrial applications. In my own years in a lab, I recall every order of pyrrole coming with extra caution stickers, not because it’s flashy, but because people know things can get interesting very quickly once the bottle opens.
Pyrrole brings a sharp, sometimes fishy odor, and boils at 130°C. The colorless liquid starts yellowing on air exposure, especially if not stored carefully. Its solubility shows off a bit of its chemical personality: it dissolves easily in hot water and many organic solvents, but not so much in cold water, reflecting that nitrogen ring doesn’t exactly love the cold bath. The five-membered ring sports a nitrogen with a lone pair ready to interact, making it both basic and slightly acidic, depending on the environment. Pyrrole rings often lose their strength under acidic or basic conditions, leading many handlers to prefer neutral pH. You won’t find stability if you leave it exposed; it readily oxidizes, especially under air and light.
Suppliers sell pyrrole with purity ranging from 98% up to 99.5%. Bottles typically feature hazard symbols for flammability and health risk, since inhalation or skin contact brings its own issues. Labels specify CAS Number 109-97-7, and alert handlers to storage demands—dark, cool, well-ventilated storage keeps the liquid from deteriorating quickly. Certified labs will detail shelf life, provide compatibility warnings, and regulatory compliance like REACH or GHS guidance. From personal practice, never trust unlabeled bottles in the fridge or any container that lacks clear hazard markings; pyrrole’s volatility and toxicity need respect, not corner-cutting.
Pyrrole’s most straightforward synthesis starts with the Paal-Knorr synthesis, linking 1,4-diketones and ammonia. Modern routes often use furan derivatives or succinimide, while early industrial methods went through acetonitrile with acetylene and ammonia. High yield and cleaner products drive the modern methods, cutting out byproducts. Each route still runs into challenges—impurity build-up, need for anhydrous conditions, and careful temperature control. In my experience, side-products show up if you become impatient; so even with better methods, attention remains the currency of quality.
Pyrrole’s five-membered ring makes it an eager participant in a host of chemical transformations. Electrophilic substitution dominates, with bromination, nitration, and formylation being common lab exercises. It slaps on substituents like a pro at the 2- and 3-positions. The ring also opens to cyclization, hydrogenation, and oxidation chemistry, showing off its potential as a creative tool for organic synthesis. Its nitrogen allows N-alkylation—a classic move to produce derivatives like N-methylpyrrole. Synthetic chemists often look for routes to porphyrin rings through careful condensation steps. Whenever my group looked for a versatile starting reagent, pyrrole offered a simple entry to complex structures, though patience sometimes wore thin when yields dipped from side reactions.
Researchers and suppliers list pyrrole under several names. Its common synonym, azole, pops up in older literature. The IUPAC calls it 1H-pyrrole. Pharmacopeias and catalogs list it under “pyrrol,” “Azole,” or “pyrrolidine precursor”. Brand-specific trade names sometimes appear, but these rarely stray far from the basic term. When ordering or searching for safety data, double-check the CAS number or chemical structure, not the marketing label.
Pyrrole belongs on the shelf with respect and planning. The toxicity isn’t the highest among chemicals, but exposure can irritate skin, eyes, and lungs. Breathing in vapors leads to dizziness and headache, and higher exposures threaten central nervous system health. Labs commit to fume hood use, gloves, eye protection, and tightly-sealed storage. Pyrrole burns quickly; any spark can set it off, especially with open containers. I’ve seen safety incidents go south in seconds after researchers skipped basic handling—one lapse almost cost a colleague their eyesight. Spills demand immediate action with absorbents and proper disposal, not improvisational cleaning. Occupational standards draw clear boundaries on workspace exposure limits, and regulatory bodies update guidance regularly.
Pyrrole carves out its spot in pharmaceuticals, agrochemicals, and specialty material science. Drug discovery relies on pyrrole frameworks for anti-inflammatory, antifungal, and anti-cancer leads. Crop science borrows the ring in designing new pesticides and growth regulators. Conductive polymers, such as polypyrrole, bring the molecule’s flexibility to batteries, sensors, and antistatic coatings. Functional dyes, catalysts, and even novel magnetic materials trace their innovation back to the utilities provided by this five-membered ring. Bio-medicine still looks to pyrrole for modeling metabolic pathways. I’ve witnessed firsthand a dramatic jump in product innovation after switching to pyrrole-based intermediates—quality climbed, costs dropped, and the lab noticed fewer waste headaches.
Much of the modern research looks at tweaking the pyrrole ring for greater efficacy in medicinal applications. Chemists invest energy in constructing pyrrole-inspired scaffolds to disrupt cancer cell pathways or create more environmentally sound agricultural compounds. Material scientists now harness polypyrrole for flexible electronics and medical devices given its biocompatibility and conductivity. Research funding sees steady increases, with new journals highlighting advances in pyrrole-based product synthesis, greener manufacturing, and medical breakthroughs. In my own lab days, the most exciting breakthroughs often started with unconventional modifications to the pyrrole nucleus, usually egged on by someone noticing “just a little difference in reactivity.” That small difference sometimes led to game-changing properties.
Long-term pyrrole exposure acts as a quiet threat. Studies reveal that chronic inhalation impairs liver and kidney function in animal models. The NIHL recommends strict airborne concentration limits, recognizing the volatile nature of this liquid. Pyrrole metabolites sometimes generate free radicals, raising oxidative stress in tissues—a trend seen in rat and mouse studies. Researchers continuously evaluate the safety margin for pyrrole’s pharmaceutical uses, especially its breakdown in the human body. Safety protocols evolve based on new animal and cell data, and regulatory authorities flag any discovery for quick review. Having watched risk assessments change as more data came out, I can say that open and honest sharing of new toxicity findings often makes the difference between lab safety and disaster.
Pyrrole isn’t about to fade into chemical obscurity. The next few years could see its derivatives pop up in more targeted therapeutics, renewable energy platforms, and smarter sensor arrays. Its potential for neural interface materials and selective drug delivery already has researchers circling around. The coupling of pyrrole chemistry with artificial intelligence in drug discovery only makes the future more interesting. Sustainable sourcing and “green chemistry” reaction routes get industry leaders thinking of pyrrole as a future-friendly compound. As someone who still keeps an eye on the chemistry journals, I can see interest in pyrrole derivatives growing. Investment will likely push new bioactive molecules from concept to clinical application, with scads of publications hinting pyrrole has more surprises left to offer.
Pyrrole doesn’t ring the same bells as silicone or nylon, but it quietly does some heavy lifting in the science world. Most chemists first cross paths with this five-membered ring in an undergraduate organic lab, usually when they’re drawing the backbone of more famous compounds. I remember sitting in lecture halls, scribbling the formulas of heme and chlorophyll, both sporting a core built from pyrrole units. One connection leads right to our blood, another to the green in plants. It’s not just a neat fact—pyrrole sets the stage for life’s basic chemistry.
Medicinal chemists covet pyrrole for one simple reason: this small ring mimics the structure of living systems. Drug makers use pyrrole to anchor molecules that treat infections, inflammation, or even cancer. Take the anti-inflammatory medicine tolmetin, or the antifungal agent ketoconazole. Both draw their punch from a pyrrole substructure. Even a slight tweak to a pyrrole ring can boost a drug’s strength or cut down on nasty side effects. Watching new medications hit the shelves gives a quiet thrill; often, these advances trace back to a dive into aromatic chemistry, trying to find the right ring for the right job.
Pyrrole keeps showing up in unexpected places. Think about the world of organic electronics. Here, scientists build polymers that carry electricity—wires you can bend, batteries built from carbon instead of copper. Polypyrrole ranks among the earliest organic conductors. Layering or spinning polypyrrole leads to new ways to make sensors, wearable electronics, or even low-cost solar cells. It’s tough to overstate the change this brings: flexible, organic gadgets rewrite the rules for design and accessibility. In my own experience, working with polypyrrole wasn’t just academic; it linked high school experiments to next-generation technology.
Dyes built on pyrrole give vivid reds and purples. Textile and ink industries rely on such molecules because they stay bright under light and stand up to washing. Pyrrole-based pigments also show up in paints and coatings. Every time color persists despite sun, rain, or cleaning, there’s a good chance a nitrogen-rich ring is anchoring that strength. The durability speaks to chemistry’s ability to meet everyday needs without asking for fancy equipment or rare minerals.
While pyrrole offers a toolbox for all kinds of industries, some issues deserve calling out. Working with pyrrole in the lab can raise safety concerns. Pyrrole itself isn’t a gentle compound—it can irritate skin and eyes, and long-term exposure does nobody any favors. In factories, these risks multiply when batches run into hundreds of liters. Engineers and workers can’t ignore the need for strong ventilation and good containment. I’ve worn more than one set of gloves thanks to this molecule.
There’s also the environmental side. Many chemical reactions used to build pyrrole derivatives produce waste that takes effort to clean. Finding greener synthetic routes—less solvent, safer reagents—makes a real difference for industrial chemistry’s future. Universities now push green chemistry as a core value, and even small improvements in pyrrole technology ripple outward to the end user, the environment, and the supply chain.
Pyrrole won’t become a household word, but in labs, factories, and even the patchwork electronics of tomorrow, it keeps earning its place. Real progress will come from the way researchers and industries handle both the molecule itself and the bigger picture—safety, sustainability, and innovation all at once.
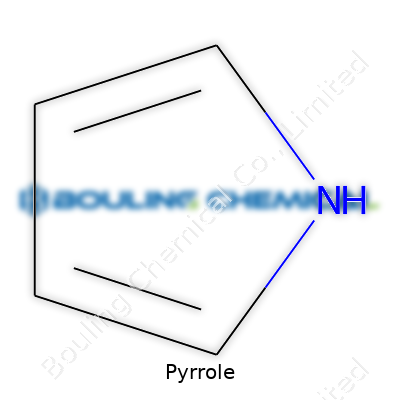
It’s easy to let chemistry drift into the realm of abstractions, especially with topics like chemical structures. Pyrrole gives us an example where structure really matters, not just for textbook completeness but for countless real-world applications. Pyrrole has a five-membered ring—four carbon atoms and one nitrogen atom—joined in a tight, almost flat cycle. There’s a single hydrogen hooked to the nitrogen. Those who’ve stared at line-and-dot diagrams recognize its alternating double bonds. That’s what chemists call an aromatic system, which basically means the electrons take a communal approach. They circle the ring in a delocalized cloud, providing stability and reactivity all at once.
In university labs, professors love wheeling out pyrrole as a “classic heterocycle.” I never saw it as just another molecule. In my mind, pyrrole trades on its simplicity—just five atoms in the ring, but the possibilities multiply fast. If you look at its applications, pyrrole refuses to stay boxed in by just being a lab curiosity. It shapes pharmaceuticals, dyes, conductive polymers, and even plays a part in what makes hemoglobin work. I remember my organic chemistry classmates wrestling with pyrrole’s electron-rich nature. That single nitrogen tucks in a lone pair, feeding into the ring’s electron system, giving it a specific fingerprint. This detail isn't just trivia—it guides reactions, like electrophilic substitution, which I learned about through late-night study sessions before tough exams.
Digging into the chemical world, pyrrole’s geometry and electron set-up step up in more corners than people expect. In pharmaceuticals, it lays down the backbone for drugs that treat a wide range of conditions, from inflammation to infections. If you ever handled polypyrrole-based films in a research project, you’d know they are flexible, conductive, and ready to get shaped for things like artificial muscles or sensors. Pyrrole doesn’t just flex its muscles in medicine or tech. Chemists draw from the ring’s ability to take on new attachments—modifications at its carbon atoms can yield colorants for clothes or agents that sneak into the active sites of enzymes.
One issue keeps popping up—making pyrrole-based compounds at large scales means facing hazardous conditions. The nitrogen’s lone pair makes the ring reactive and sometimes unpredictable, especially during manufacturing. This risk calls for more research into gentler catalysts, safer conditions, or perhaps whole new ways of building the ring, like using renewable feedstocks. I think a change in perspective—seeing pyrrole as both an opportunity and a challenge—opens space for innovation.
Chemists have already started to harness greener methods, but there’s more work on the horizon. Collaboration across fields—synthetic chemistry, engineering, and even AI-driven design—may set up safer, more eco-friendly processes. Some startups even offer biotechnological routes to heterocycles, promising less waste and lower emissions. That takes pyrrole from the bench to broader impact.
Recognizing pyrrole’s chemical structure isn’t just about memorizing the placement of atoms. It forms the basis for real decisions in research and industry. Every new application—whether electronics, medicines, or pigments—builds on a solid grasp of the way pyrrole’s atoms lock together and share electrons. I’ve seen students and researchers light up when they realize the ring isn’t just a diagram—it’s the key to unlocking practical solutions to hard problems.
Pyrrole floats under the radar in most conversations unless you regularly mix chemicals for a living. In the world of organic compounds, it appears as a colorless volatile liquid with a smell that reminds me of chloroform, something you probably never want to sniff up close. Chemists prize it for building bigger molecules—pharmaceuticals, dyes, even some plastics—so this stuff pops up in more places than most folks think.
Step into any high school chemistry class and you’ll remember those bright yellow “toxic” stickers on bottles behind locked cabinets. Pyrrole doesn't come with a Hollywood-style danger sign, but treating it lightly leads to trouble. A big issue comes from its high flammability. Spill a few drops near a heat source, you’ll get a quick lesson in why chemical storage matters. Vapors can catch fire at room temperature, a headache for labs and factories everywhere.
Then there’s the health side. Inhaling pyrrole makes breathing hard and brings on headaches, nausea, even dizziness. Long exposure in labs with poor ventilation leaves workers tired and irritated, both in their airways and their attitude. Spills on skin bring redness and itching. Not as dramatic as Hollywood toxins, but enough to push anyone to grab gloves and goggles.
Factories using pyrrole dump very little into the environment, especially where countries enforce chemical rules. Strict disposal rules mean most companies keep it under control. Still, accidental spills happen, and when they reach soil or water, they can cause local harm. Animals and plants exposed directly can suffer, though large-scale disasters remain rare. As a parent, knowing what might leak into a water supply turns worry into action. Stories of chemical fires or dumping have pushed whole communities to demand better accountability.
Looking at it from an everyday person’s view—the riskiest encounters show up in workplaces making drugs or using specialty paints. Rarely will you stumble across pyrrole at home or in the supermarket, unless you work in a specialized industry. Most folks trust that labs and companies stick to safety law, but trust wobbles when headlines talk about toxic leaks or worker injuries.
Safer use starts with information. Many accidents link back to poor training or old equipment. Companies focusing more on refresher courses and better storage have cut injuries. Safety data sheets for pyrrole now spell out its vapor dangers, fire risk, and first aid steps clearer than ever. Strong ventilation, regular air checks, and fire-proof storage rooms turn theory into real-world safety. I’ve watched workplaces add more sensors and alarms—not a perfect fix, but it keeps the odds in workers' favor.
Pushing industry to use less hazardous solvents pays off in the long run. Researchers keep hunting for safer chemicals that still get the job done. Some new synthetic techniques swap out pyrrole entirely, though switching costs time and money. Consumers want cleaner products, investors don’t want lawsuits, and managers breathe easier with fewer close calls. Often, it’s shared experience—someone’s accident story—that drives meaningful change faster than written policy.
Pyrrole can be dangerous, especially without good safety steps. Companies and labs facing it daily know well that respect and careful handling save headaches, literally and figuratively. Pushing for safer alternatives and strong education prevents most serious trouble. For everyone else, just knowing who uses what chemicals—and how they keep workers safe—keeps communities honest and healthy.
Messing around with organic chemistry in college, I remember the peculiar smell of pyrrole long before I really understood how it forms. It's no secret among chemists that such five-membered rings open new doors for everything from medicine to pigments. The story of its creation stretches far back, running through textbooks and time-tested beakers.
In classrooms and on production floors, people don’t waste effort reinventing the wheel. Out of all the tried and true options, the Knorr and Hantzsch syntheses stand out. Take the Knorr method: it relies on β-ketoesters and ammonia or primary amines. Mix these with a dash of acid, then heat them, and you get your pyrrole ring. What makes this interesting isn’t the neatness of the route but how accessible the materials are. There’s a reason this method hangs around in undergraduate labs and industrial setups.
Experience shouts louder than theory. The first time I watched a failed pyrrole synthesis, it came down to moisture ruining the reaction. Unlike some tougher compounds, pyrrole springs up best in dry settings. Throw in some water and you end up scraping gunk out of flasks instead of analyzing clean crystals. This lesson pushes everyone to double-check glassware and crank up the vigilance, at least if they're hoping for good yield.
People rarely think about where their hair dye, antibiotics, or agrochemicals start life. Countless consumer goods trace back to this little heterocycle. Lab chemists and manufacturers value pyrrole syntheses that keep costs manageable and outputs consistent. This drives innovation: researchers test catalysts, greener reagents, and milder temperatures, hoping to make production safer and easier to scale up.
Convincing any company to shake up routines isn’t easy. Safety concerns come up again and again, especially once you scale up from milligrams to kilograms. Ammonia stings the eyes and some intermediate compounds don’t belong outside a well-ventilated hood. Factories spend money on scrubbing emissions and training staff to keep both people and the environment in the clear. Green chemistry steps in, so new research leans toward less-toxic solvents and recyclable catalysts.
People are hungry for answers that cut cost, energy, and risk all at once. Some scientists tweak classic reactions to reduce byproducts. Others draw on flow chemistry—pumping reactants continuously through tubes—hoping for more control and less waste. I’ve seen young chemists’ eyes light up at the idea of running a complex reaction without the drama and mess of old school batch setups. Though the lab books don’t always spell this out, motivation often starts with a ruined reaction and a clock racing toward deadline.
Behind every smooth-running process sits someone who found a workaround for a stubborn reaction. As consumers, we rarely think about the chain of steps it takes to build a useful compound like pyrrole. For the folks in the lab, every improvement—whether it makes reactions safer, cheaper, or cleaner—pays off in jobs, new products, and, sometimes, fewer headaches at the end of a long day.
Pyrrole isn’t a substance that most folks will see stacked up in storage closets, but its unique nature means it demands smart attention. The liquid itself looks unassuming. It’s colorless when pure, but it usually arrives with a yellow tinge because it doesn’t like being exposed to air or light. Pyrrole tends to oxidize fast, turning brown if left out or stored poorly. In the lab, it’s almost a rite of passage to find an old bottle turned sludgy, teaching a quick lesson about poor storage habits.
Keeping out air isn’t just about avoiding mess. Pyrrole reacts with oxygen. That reaction starts off slow—you might not catch it straight away—but over days, things thicken up. The material can gum up pipettes, clog containers, and, worse, break down into stuff that’s both toxic and unpredictable. Ultraviolet light speeds things up far more than most realize. Sunlight through a window, or even the wrong lab lighting, can wreck a whole batch.
Heat ramps up these problems. Pyrrole boils at just over 130°C, but trouble starts long before that. At room temperature, you already see it degrade faster with any warmth. Someone stacking chemicals near a radiator or on a sunny shelf asks for waste, fumes, and sometimes headaches from the breakdown products.
A basic, air-tight glass bottle, topped off with a solid rubber or Teflon-lined cap, gets you halfway there. I’ve learned not to trust plastic stoppers. Many plastics let gases in, slowly but surely—or let small amounts of pyrrole escape. Using amber glass bottles keeps out most light. You don’t always need total darkness, though wrapping the bottle in aluminum foil helps protect it in sketchier locations.
Keeping pyrrole cold slows any reaction. A fridge at two to eight degrees Celsius works well, though some labs drop it into minus twenty-degree freezers for longer stretches. Sealing the container under nitrogen or argon gas fixes much of the oxidation risk. It feels like overkill at first, but after tossing ruined bottles, that extra precaution saves money and hassle.
Plenty of focus lands on keeping pyrrole pure—rightly so. But rarely do storage guides highlight the smell. Pyrrole stinks, even in small leaks, and working in a cramped storeroom with a whiff of it can make anyone queasy. Good ventilation isn’t a luxury here, it’s basic survival during cleanup. Over time, inhaling vapors brings its own health risk, adding one more reason not to cut corners.
Labs that ignore these guidelines see their supply budgets balloon. Pyrrole stored in open or half-sealed bottles, or in a warm spot, spoils fast and collects moisture to boot. This isn’t just an issue for syntheses—it can muck up sensitive instruments, and it’s tricky to work with tainted material. One contaminated sample can set off a bad chain reaction in a shared environment, risking cross-contamination.
Simple practices beat out exotic fixes. Fresh containers, nitrogen or argon headspaces, a consistent place in the fridge—and labeling containers with the date they were opened. Labs that invest in amber bottles and a designated cooled storage area see fewer headaches. For anyone working with pyrrole, these steps don’t take long; they just protect people, budgets, and research outcomes. Keeping it clear and safe isn’t about fear—it’s a nod to experience, backed by plenty of ruined bottles and a few too many headaches.
| Names | |
| Preferred IUPAC name | 1H-pyrrole |
| Other names |
Azole 1H-Pyrrole Pyrrol |
| Pronunciation | /ˈpɪr.oʊl/ |
| Identifiers | |
| CAS Number | 109-97-7 |
| 3D model (JSmol) | `Pyrrole|1|C|2|N|1,2|2,3|3,4|4,5|5,1` |
| Beilstein Reference | 1209221 |
| ChEBI | CHEBI:35589 |
| ChEMBL | CHEMBL14138 |
| ChemSpider | 7281 |
| DrugBank | DB03261 |
| ECHA InfoCard | 100.007.568 |
| EC Number | 201-830-4 |
| Gmelin Reference | 726 |
| KEGG | C01742 |
| MeSH | D011693 |
| PubChem CID | 8026 |
| RTECS number | UQ1050000 |
| UNII | Y1C935KY7T |
| UN number | UN1265 |
| Properties | |
| Chemical formula | C4H5N |
| Molar mass | 67.09 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | tar-like |
| Density | 0.967 g/mL |
| Solubility in water | slightly soluble |
| log P | 0.72 |
| Vapor pressure | 3.54 kPa (at 20 °C) |
| Acidity (pKa) | 16.5 |
| Basicity (pKb) | 13.6 |
| Magnetic susceptibility (χ) | −18.0 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.509 |
| Viscosity | 0.88 mPa·s (at 20 °C) |
| Dipole moment | 1.74 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | S⦵298 = 274.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 86.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1594 kJ/mol |
| Pharmacology | |
| ATC code | D04AA33 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02, GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H302, H312, H315, H319, H332 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P304+P340, P305+P351+P338, P370+P378, P403+P235 |
| NFPA 704 (fire diamond) | 1-2-0-S |
| Flash point | 31 °C |
| Autoignition temperature | 200 °C |
| Explosive limits | 1.8–9.6% |
| Lethal dose or concentration | LD50 oral rat 1214 mg/kg |
| LD50 (median dose) | LD50 (median dose): 359 mg/kg (rat, oral) |
| NIOSH | NIOSH = "QK8400000 |
| PEL (Permissible) | PEL: 15 ppm |
| REL (Recommended) | 5 ppm |
| IDLH (Immediate danger) | 400 ppm |
| Related compounds | |
| Related compounds |
Pyrroline Pyrrolidine Indole Pyrrole-2-carboxylic acid |