Curiosity about pyrrole compounds began over a century ago. Chemists in the late 1800s, always keen to poke holes in carbon rings just to see how these skeletons played together, isolated pyrroles from natural sources such as bone oil. Later generations started tweaking the pyrrole nucleus to see what would happen. Pyrrole-2-carbaldehyde popped up as a particularly active player, especially once researchers understood its curious blend of aromaticity and reactivity. In the 1900s, commercial labs began looking at this aldehyde for its use in flavor, fragrance, and, later, pharmaceuticals. This journey hasn’t always followed a clean-cut path. At different times, regulators and businesses have doubled back, testing its safety or weighing environmental impact, particularly as chemists branched off into heterocyclic aldehydes for drug discovery.
Out of all the substituted pyrroles, Pyrrole-2-Carbaldehyde offers a distinctive nutty, earthy aroma that snags attention in the world of natural flavors. Many users come across it in low concentrations in cooked foods such as bread crusts or roasted coffee. The chemical rarely arrives alone. Batches will almost always carry neighboring impurities from synthesis or breakdown, which means attention to detail during manufacture and storage becomes crucial for both researchers and commercial users—whether in fine chemicals, perfume ingredients, or new medicinal intermediates.
Anyone who’s worked with Pyrrole-2-Carbaldehyde in a lab won’t soon forget its pungent, slightly sweet-vegetal smell. This yellow-brown liquid melts below room temperature and boils in the mid-200°C region. Its modest solubility in water, which typically clocks in at a few grams per liter, contrasts with its much happier performance in polar organic solvents like dimethylformamide or ethanol. Once exposed to air, the aldehyde group can slowly oxidize, so work often goes faster when labs flush glassware with nitrogen. The aromatic pyrrole ring coupled with an electron-hungry aldehyde means this molecule pulls double duty in synthesis: it easily enters condensation reactions but sometimes surprises with side reactions due to its sensitive hydrogen atoms, especially in the ring.
In a chemical supply catalog, Pyrrole-2-Carbaldehyde shows up with detailed purity calls and impurity profiles, since even tiny traces can interfere with flavor use or fine pharmaceuticals. Reliable sources offer at least 96% purity, often higher for pharmaceutical or analytical work. Transport and storage require secure, airtight amber bottles, with labeling that flags irritancy and the requirement to keep containers below 25°C. The Safety Data Sheet (SDS) tells handlers to avoid inhalation and skin contact, and includes emergency spill and fire procedures, as even minor exposure can trigger eye and lung irritation.
Chemists tend to favor the Vilsmeier-Haack reaction for preparing Pyrrole-2-Carbaldehyde, thanks to its reactivity and the clean hand it plays with the pyrrole ring. In this process, N,N-dimethylformamide and phosphorus oxychloride come together to generate an electrophile, which attacks pyrrole selectively at the 2-position, then reveals the aldehyde group on workup. Variations pop up—some swap in milder chlorinating agents, others run the reaction in greener solvents to ease waste handling. Yields commonly hit above 60%, though side reactions urge operators to constantly check temperature and dump in plenty of cooling.
Pyrrole-2-Carbaldehyde’s story doesn’t end at synthesis. The aldehyde can swing into action in a slew of coupling reactions. Chemists often build up more complex heterocycles or jazz up natural origins by tacking on bigger, more elaborate groups. Through classic Knoevenagel or Claisen-Schmidt condensations, it latches onto other aromatic systems, lending a synthetic backbone for pharmaceutical candidates or advanced materials. The exposed ring can be halogenated for electronic device precursors or can undergo formyl group reductions to produce simpler derivatives. Each new molecule gets tested for properties well beyond simple flavorings: biological activity, electronic features, or thermal stability, depending on where curiosity or commerce directs research.
In catalogs and research papers alike, Pyrrole-2-Carbaldehyde sometimes appears as 2-Formylpyrrole, 1H-pyrrole-2-carboxaldehyde, or 2-pyrrolecarboxaldehyde. The pharmaceutical world and flavor chemists both keep these synonyms handy to avoid confusion across international suppliers and regulatory filings. Each alternate name still identifies the same key functional group—a formyl at the 2-position of the pyrrole core—which means users don’t need to worry about structural changes hiding behind the label.
Lab vets know better than to dismiss the hazards posed by aromatic aldehydes. Pyrrole-2-Carbaldehyde’s vapor irritates eyes, nose, and lungs in short order, triggering sharp coughing or watering eyes in unprepared lab mates. Prolonged handling or spills turn the skin red and sore. Regulations demand solid personal protective equipment: gloves, goggles, and a working fume hood. Disposal of waste, often rich in halogenated solvents or minor tar, gets routed through specialized destruction, never tossed down the drain. In scaling up for pilot plants or bulk manufacture, process safety reviews flag the need to limit emissions, lock-in storage protocols, and ensure emergency equipment stands ready—not just for chemical fires, but for escape from accidental inhalation.
Wherever complex molecules spring up—from crude flavors to high-stakes pharmaceuticals—Pyrrole-2-Carbaldehyde often plays a pivotal part. Flavor specialists extract it from heated foods, or blend it into synthetic mixes to give coffee, chocolate, or roasted foods a lift in aroma. Drug hunters turn to its reactivity for stitching together potential antibiotics, anti-inflammatory agents, or new small-molecule scaffolds. Even cutting-edge materials science looks at its derivatives for organic electronics or sensors. The versatility of this single functional group drives wide adoption, even as individual industries refine purity and analytical techniques to keep their products safe and effective.
University and industrial research teams have not finished mapping Pyrrole-2-Carbaldehyde’s full potential. Recent years brought a surge in medicinal chemistry, as scientists seek molecules to sidestep antibiotic resistance or push into new therapeutic classes. Electrochemical techniques now measure its behavior in solar cell or battery designs, with researchers tuning ring substitutions for better charge mobility. Open-access data and machine learning throw light on how subtle tweaks alter bioactivity or environmental persistence, letting research keep pace with demands from sustainability and green design. Manufacturers, in turn, challenge themselves to synthesize this intermediate with less waste and greater atom efficiency, responding to tightening regulations on hazardous by-products.
Toxicologists have flagged Pyrrole-2-Carbaldehyde for its reactivity, which raises both opportunity and risk. Cell culture and small-animal studies show mild to moderate acute toxicity, particularly by inhalation or prolonged skin exposure. Rodent testing has mapped out safe exposure limits, while long-term studies still run to settle questions about possible chronic effects. Regulatory agencies tend to set occupational limits at low parts per million. Much like other aromatic aldehydes, this compound can form reactive intermediates in the body, so researchers keep investigating its metabolic fate and potential to form protein adducts linked to hypersensitivity or immune response.
Not much about Pyrrole-2-Carbaldehyde feels stale. Its chemistry opens doors for novel drug entities, advanced sensors, and upcycled flavors using cleaner, greener synthesis pathways. Industrial adoption will likely hinge on safer production protocols and tighter regulatory compliance, as sustainability rules push manufacturers to reduce both waste streams and hazardous reagent use. Digital modeling and AI-driven laboratory automation have already begun to shape new research paths, helping chemists shorten the time between discovery and application while tracing toxicological impact earlier in the pipeline. Whether in flavors or pharmaceuticals, the future for this molecule—like so many crafted through persistent curiosity—rests with those who balance reactivity with responsibility.
Pyrrole-2-Carbaldehyde sounds like something hidden away in a dusty corner of the chemistry lab, but its impact shows up in many areas. This molecule reflects the kind of unsung workhorse chemistry often delivers—quietly shaping things we see and use every day.
The search for better medicine often starts with small tweaks to well-known structures. Pyrrole-2-Carbaldehyde plays a role in making new pharmaceuticals. Scientists use it to anchor parts of more complex drugs, especially those aimed at fighting infections or controlling inflammation. A friend who researches antimicrobial agents once explained that the structure of pyrrole rings provides a natural starting point for building blocks in these compounds. Published studies back this up, showing how compounds built from pyrrole-2-carbaldehyde show promise against bacteria and fungi that have grown resistant to older drugs. The process of turning a small molecule into a life-saving drug takes dedication, and pieces like pyrrole-2-carbaldehyde drive that progress forward.
Pyrrole-2-Carbaldehyde also pops up in materials science research. Building better polymers—those flexible plastics and coatings that show up in packaging, electronics, and everyday tools—often takes precision. Chemists harness the reactive nature of pyrrole-2-carbaldehyde to design smarter, tougher, or more specialized materials. In my experience working with research teams, small changes to a molecule’s structure can lead to coatings that last longer under harsh conditions, inks that conduct electricity for the next generation of flexible electronics, or even scaffolds for tissue growth in medicine. Scientific journals highlight the molecule’s role in new conductive polymers, expanding possibilities in electronics and energy storage.
The world of flavors and fragrances leans on molecules that pack a punch, even in tiny amounts. While pyrrole-2-carbaldehyde isn’t a household name, its derivatives help shape the savory, nutty, or toasted notes in certain foods. Developing a new snack or beverage often depends on recreating those rich flavors naturally found in roasted foods. A food scientist once described how tweaking simple compounds creates the right aroma for chocolate or coffee. This molecule’s structure helps experts build more attractive flavors, pushing the boundaries of plant-based and low-cost foods. Its impact stretches from the chemistry book to the dinner table, even if most consumers never spot the name on a label.
Leadership in innovation depends on access to versatile building blocks—chemical ingredients that open doors to new discoveries. Pyrrole-2-carbaldehyde delivers because it blends reactivity with stability, making it easy to work into larger, more advanced molecules. Environmental and safety concerns keep growing every year. Researchers look for newer ways to produce and recycle these compounds safely, to reduce waste and exposure without slowing down scientific progress. Policy groups, universities, and industry groups now share data on its safe handling, signaling that knowledge really can lead to safer outcomes for everyone involved.
Every so often, a simple molecule starts a chain reaction—helping drive breakthroughs in medicine, materials, and everyday comforts. Pyrrole-2-carbaldehyde may not get headlines, but its versatility keeps labs busy and holds possibilities for the world outside them. Years of research prove that even small changes can deliver big benefits, and this molecule keeps proving its worth.
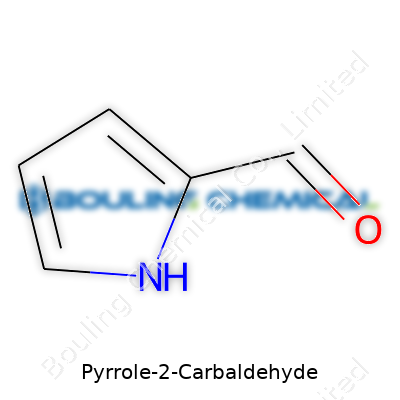
Pyrrole-2-carbaldehyde holds a chemical formula of C5H5NO. It stands out as an aromatic heterocyclic aldehyde. The aldehyde group attaches itself right next to the nitrogen in the five-membered pyrrole ring. So, let’s talk structure for a second: You’ll see four carbons, one nitrogen, five hydrogens, and one oxygen. It’s not just a string of atoms—each piece shapes its role in chemistry and biology.
Lab work has shown me that small molecules like this can punch far above their weight. Pyrrole-2-carbaldehyde pops up in medicinal chemistry circles. It crops up in research on antifungal, antibacterial, and even anti-cancer compounds. Chemists take advantage of its reactive aldehyde group to build bigger, more complex molecules.
The arrangement of atoms carries a lot of meaning. Aromatic rings, especially ones containing nitrogen, interact with other molecules in the body in ways that regular hydrocarbons can’t. Medicinal chemists look for these sorts of structures because tiny changes at the molecular level can set the stage for important breakthroughs.
The importance isn’t limited to medical research. Organic synthesis labs lean on versatile compounds for routes to new materials. I’ve spoken with researchers who use pyrrole-2-carbaldehyde to create heterocyclic frameworks for dyes and polymers. Materials science doesn’t have much patience for one-trick ponies, and this compound finishes reactions cleanly and without too much fuss, making it popular for scale-up.
Anytime a molecule enters environments that connect to health and medicine, the stakes go up. Pyrrole-2-carbaldehyde sits in a class of chemicals that need careful handling. Short-term contact can lead to irritation; breathing its vapors isn’t a good idea. Workspaces that manage this compound should keep ventilation and personal protection top of mind. Mistakes in safety leave permanent lessons. Good documentation and regular training matter—no one walks away from an accident feeling lucky.
Developers using this chemical in medicine must stay on top of regulatory requirements and commit to transparent science. Data need to reflect real outcomes, and researchers should avoid shortcuts in both clinical and lab work. Trust in new medicines springs from honest work.
Making new compounds only gets us halfway. Labs should share findings openly, reducing wasted effort and speeding up progress. Building collaboration between academic labs and industry shortens the leap from experiment to application. In my experience, shared data saves lives when breakthroughs emerge faster.
Education offers another missing step. Training young scientists about both the promise and pitfalls of aromatic aldehydes plants seeds for safer labs everywhere. Since chemistry doesn’t unfold in a vacuum, schools need to teach both the theory and the daily practice so students graduate ready to handle whatever comes their way.
Pyrrole-2-carbaldehyde, with its formula C5H5NO, illustrates the way a handful of atoms can shape innovation and discovery. Each new synthesis, each careful safety measure, and each transparent report builds the culture that keeps science moving forward for everyone.
I’ve worked with enough chemicals over the years to see how a small mistake can turn into a costly incident. Pyrrole-2-Carbaldehyde isn’t one of those loud, attention-grabbing compounds, but people working in labs or manufacturing shouldn’t take it lightly. Storage choices impact safety, product quality, and even regulatory compliance. Ignoring these needs can mean ruined material or, worse, putting people at risk.
Anyone who’s ever opened a container of Pyrrole-2-Carbaldehyde knows the strong, unpleasant odor. That smell comes with volatility. This chemical reacts easily with air and light, and it can turn unstable or degrade if humidity creeps in. If someone stores it wrong, they’ll probably end up with something yellowish or brown, not the clear liquid they bought. That can mess with experiments or finished products. In my experience, a ruined reagent can put a halt to days of work.
Pyrrole-2-Carbaldehyde also poses fire risks because it gives off flammable vapors. I have seen labs where a simple spark, or a hot surface nearby, was all it took to turn an overlooked bottle into a potential hazard.
Storage really starts with the container. A tightly sealed amber glass bottle goes a long way. The amber glass blocks light, which helps slow down degradation. A loose cap lets vapors escape, and pulling in air invites moisture and oxygen into the mix. That increases the risk of turning good chemical into useless sludge, and it’s happened more than once in labs I’ve worked in.
I always recommend keeping Pyrrole-2-Carbaldehyde in a cool spot. Room temperature might seem fine, but even mild heat speeds up that chemical’s breakdown. A refrigerator set between 2°C and 8°C usually helps, unless there’s risk of freezing. I once saw someone freeze a whole batch by accident—glass shattered, product ruined. So label everything and check temperature settings often.
A dry environment matters. Moisture is more destructive than many realize—humidity in the storage room can turn Pyrrole-2-Carbaldehyde into a gummy mess. Simple silica gel packs in the cabinet keep water at bay, and checking for leaks or spills helps avoid cross-contamination. I’d always wipe down bottles to keep things clean. Less water and less dust means longer shelf life.
Every facility handles chemicals a bit differently, but laws and rules put worker safety at the center. Safety Data Sheets from suppliers give specific tips. For Pyrrole-2-Carbaldehyde, these always spell out no open flames, no static sources, and immediate cleanup of spills. Mandatory training covers personal protective equipment like nitrile gloves and goggles. I’ve seen places skip steps with less obvious chemicals, only to deal with headaches later—literally and figuratively.
Labeling bottles with purchase dates, expiration dates, and hazard warnings takes only a moment but prevents mistakes—especially for new staff coming into a busy lab or plant. Secure storage also means locked chemical cabinets if there’s any risk of unauthorized access. That keeps everyone above board with local laws and gives peace of mind.
Better training, good habits, and careful monitoring all add up. I’ve found that storing Pyrrole-2-Carbaldehyde correctly not only protects property but keeps people safe. It’s not about being paranoid or following rules for the sake of it, but about respecting a powerful tool that can make or break a project.
Pyrrole-2-carbaldehyde, found in some laboratory settings and used in chemical synthesis, gives off a sharp, sometimes pungent odor. Its appearance doesn’t stand out, looks a bit like other organic chemicals, but its chemical structure raises some questions for people who aren't regularly dealing with specialty chemicals.
Looking at the material safety data from trusted chemical suppliers, this compound doesn’t appear as hazardous as strong acids or corrosives. Still, it brings enough concerns to make anyone handling it pay attention. Pyrrole-2-carbaldehyde can irritate the skin, eyes, and respiratory system. There’s real evidence, not just lab speculation. If you breathe in its vapors or have skin contact, expect some discomfort or redness. I’ve seen colleagues accidentally brush a gloved hand across their face after handling similar compounds; even small exposures resulted in red eyes and nasty irritation.
Toxicity data, while not as detailed as with more commonly used chemicals, show that ingesting even a small amount causes damage. A study exploring its acute oral toxicity in rodents pointed to liver and kidney distress. These organs work overtime to filter out strange chemicals, and pyrrole-2-carbaldehyde puts pressure on the system. I trust the findings from the National Center for Biotechnology Information highlighting increased stress markers after exposure—it's a wake-up call for those who think ordinary gloves and a basic fume hood offer full protection.
One point that stands out: chemical reactivity. Pyrrole-2-carbaldehyde reacts with strong oxidizers and acids. This isn’t just theoretical; poor storage or accidental mixing in a cluttered lab can kick off fires or create noxious fumes. Years back, an inattentive intern stored it with peroxides. They got lucky—no explosion—but we all learned a lesson about separate shelves for a reason. Fire marshals and safety officers always stress the basics, but these stories show that chemistry can turn an oversight into a crisis.
Unlike table salt or sugars, this chemical brings a low threshold for inhalation and skin exposure. The Occupational Safety and Health Administration reminds us that airborne solids or droplets should always count as a hazard, even in a well-run facility.
Gloves, splash goggles, and lab coats hardly feel excessive for this kind of work. Even if someone moves fast and only for a moment, the risks stay real. Fume hoods matter—there’s no safe shortcut for indoor environments. Chemical spill kits tailored for organic solvents and access to emergency showers matter just as much as the gear itself.
For storage, separate this compound from strong oxidizers and acids—no excuses. Lock it in a well-labeled cabinet, out of regular reach, away from food and common supplies. Training goes a long way: staff need to recognize the smell and handle spills right away, not later. All of this cuts accidents and exposure to nearly zero.
The takeaway is simple: pyrrole-2-carbaldehyde isn’t something to panic about, but it's not your average cleaning product either. You see the impact it could have, especially in poorly ventilated labs or places skipping important safety steps. Trusting labels, using the right protective equipment, and taking chemical reactivity seriously is how professionals avoid injury or property damage. If researchers and students treat this compound with the respect it deserves, risk melts away. In the end, that’s good for the lab, the workers, and anyone else walking the hallways.
Walk into a research lab or step onto a plant floor, and there’s this constant search for chemical ingredients that quietly make a big difference. Pyrrole-2-carbaldehyde sits in that corner of useful but rarely bragged-about compounds. Scientists know it as an organic building block with a knack for sparking new ideas in both research and industry. It doesn’t make headlines, but it keeps showing up where something new gets built.
Pyrrole-2-carbaldehyde often jumps into action when researchers want to investigate something fresh in drug discovery. In my own early-days lab experience, synthetic chemists kept small vials of it alongside better-known reagents, ready for a test on a Friday afternoon. Adding it into a reaction often spun out molecules that looked promising as antiviral or anticancer leads. Researchers go after its structure since it brings together a reactive aldehyde and a five-membered heterocycle, both of which let you snap on new fragments, building up molecular scaffolds for drug candidates. Back this up with the fact that many bioactive natural products and pharmaceuticals contain pyrrole units—pyrrole-2-carbaldehyde often acts as one of the best ways to build those skeletons.
Polymers, electronics, and dye technology each carry stories about pyrrole-2-carbaldehyde quietly anchoring something big. The electronic properties of the compound make it popular for spinning up new types of organic semiconductors, which get tested in everything from solar cell prototypes to flexible display screens. Dye chemists gravitate to it because the pyrrole ring allows for bold structural tweaks—an insight you only get after seeing a dozen side-by-side color comparisons under the lamp. Some high-performing light-sensitive dyes come about by tweaking pyrrole-2-carbaldehyde and stringing it into larger chromophores. It doesn’t just stay on the page of a journal; it ends up tied into the circuits and coatings that engineers use every day.
A scientist working on building a natural product might reach for pyrrole-2-carbaldehyde to slam through a few steps faster than usual. Its straightforward chemistry makes it a go-to intermediate for constructing both simple and tricky ring systems. Many case studies in organic synthesis publish routes where this molecule narrows reaction steps, trims down purification headaches, and keeps costs under control—assets that matter whether you’re working at the bench or scaling up for a pilot plant.
Some hurdles pop up too. Pyrrole derivatives, this one included, can break down in the open or react with just about anything if handled carelessly. In practice, that means tight control in storage, good ventilation, and routine checks on reactivity. On the environmental side, chemists keep an eye on waste and choose milder conditions, aiming for process routes where leftover materials get reused or neutralized. The green chemistry push isn’t all slogans here—it drives smarter design from early research through to large-scale production.
Whether you’re sketching out a pharmaceutical scaffold, spinning up a next-gen light-absorbing dye, or mapping a new synthesis pathway, pyrrole-2-carbaldehyde gives builders a flexible, responsive tool. It serves quietly, letting new science and manufacturing pivot toward better, safer, more creative products. That’s worth remembering when the next breakthrough or new consumer gadget traces its roots back to this unassuming little molecule.
| Names | |
| Preferred IUPAC name | 1H-pyrrole-2-carbaldehyde |
| Other names |
2-Formylpyrrole 2-Pyrrolecarboxaldehyde 2-Pyrrolealdehyde |
| Pronunciation | /ˈpɪr.oʊl tuː kɑːrˈbæl.dɪ.haɪd/ |
| Identifiers | |
| CAS Number | 872-85-5 |
| 3D model (JSmol) | `COC1=CC=CN1C=O` |
| Beilstein Reference | 1092094 |
| ChEBI | CHEBI:16118 |
| ChEMBL | CHEMBL45580 |
| ChemSpider | 137518 |
| DrugBank | DB08419 |
| ECHA InfoCard | 100.019.159 |
| EC Number | 201-856-5 |
| Gmelin Reference | 86168 |
| KEGG | C06154 |
| MeSH | D000386 |
| PubChem CID | 6947 |
| RTECS number | UJ8225000 |
| UNII | V8F1TYY6QI |
| UN number | UN3439 |
| CompTox Dashboard (EPA) | DTXSID9044363 |
| Properties | |
| Chemical formula | C5H5NO |
| Molar mass | 93.10 g/mol |
| Appearance | Yellow to brown liquid |
| Odor | aromatic |
| Density | 1.099 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 0.99 |
| Vapor pressure | 0.289 mmHg (at 25 °C) |
| Acidity (pKa) | 14.0 |
| Basicity (pKb) | 1.86 |
| Magnetic susceptibility (χ) | -38.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.553 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.92 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 325.0 J⋅mol⁻¹⋅K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -12.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1170 kJ·mol⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | F, GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements: P261, P280, P305+P351+P338, P304+P340, P405, P501 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | 50 °C |
| Autoignition temperature | 100 °C (212 °F; 373 K) |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 1260 mg/kg |
| NIOSH | Not Listed |
| PEL (Permissible) | Not established |
| REL (Recommended) | Refrigerated (2-8°C) |
| Related compounds | |
| Related compounds |
Pyrrole Pyridine-2-carbaldehyde 2-Furaldehyde Indole-3-carbaldehyde Thiophene-2-carbaldehyde |