Scientists first noticed pyrazinylethanethiol’s signature sulfur profile back in the surge of synthetic chemistry during the mid-1900s. Research into flavor compounds took off, and folks looking for ways to recreate meaty, roasted aromas stumbled on this molecule in the lab while attempting to mimic natural pyrazine derivatives found in cooked foods and roasted coffee. It marked a step forward in flavor science, pushing open doors to a new set of aroma and taste molecules ready for commercial flavor houses, perfumery, and food formulation. From those early experiments, chemists refined the molecule’s structure, stabilizing it for practical use. Decades of tweaks in synthesis and analytical technology revealed pyrazinylethanethiol’s unique charm and gave it lasting value in several industries.
Pyrazinylethanethiol brings a strong, savory and roasted scent—some call it “meaty,” others detect coffee or even onion notes. Suppliers usually offer it as a yellowish to pale liquid, sold in tiny vials because a small dose transforms the smell of a much bigger batch. It’s used most often in flavor and fragrance creation, sometimes in animal feed, and even in research as a trace marker. Beyond its punchy scent, it shows up in studies looking into how people and animals perceive subtle flavors, often acting as a standard in labs trying to understand taste and aroma at the molecular level.
Handling pyrazinylethanethiol, the nose gets hit with pungency even at low concentrations. Its boiling point’s usually in the 180 to 210°C range, with a melting point left undetected due to its liquid form at room temperature. With its dual pyrazine and thiol structure, the molecule sports moderate polarity—making it soluble in common organic solvents such as ethanol, ether, and sometimes in water if concentration drops low enough. Density hovers near 1.10 g/cm³, and it brings a refractive index between 1.53–1.56. The volatile nature means it won’t stick around in open air too long, but it lingers enough in mixtures to change a whole flavor or fragrance profile.
Product labels list the molecule along with its standard chemical name—usually 2-(Pyrazin-2-yl)ethanethiol—and CAS number (36642-66-7). Purity clocks in at over 95% for most food-grade or research-grade stocks, and batch analyses confirm absence of heavy metals and residual solvents, so regulators stay satisfied. Shipping documents include its hazard classification: labeled as a flammable liquid, with health warnings about inhalation and skin exposure. Containers lock tightly with vapor-proof seals. Professional handlers learn pretty fast to store it in cool, ventilated cupboards, far from food prep surfaces.
Most manufacturers synthesize pyrazinylethanethiol by starting with pyrazine and introducing ethyl mercaptan through alkylation reactions. This approach works because the pyrazine ring activates toward substitution, especially with an appropriate catalyst to steer selectivity for the 2-position. Additional steps quench byproducts and purify the end molecule. Some processes rely on precursor compounds made from petroleum-derived feedstocks, while others incorporate biocatalysts in newer “green chemistry” attempts. Once isolated, distillation under inert gas pulls out a clean fraction, ready to bottle and ship to flavor houses or research labs.
Chemists have explored several methods to tweak pyrazinylethanethiol’s chemical structure. Oxidation introduces sulfoxide or sulfone groups, sometimes dulling its strong aroma for novel fragrance applications. Reacting it with alkyl halides produces derivatives with longer chains, which can soften or shift the aroma profile. Acidic or basic conditions crack the molecule apart, so most users avoid strong reagents unless aiming for deliberate breakdown. Researchers often search for ways to add stabilizers or “masking agents” so the signature scent doesn’t overpower everything, and to keep the compound useful in mixtures that linger on store shelves.
Across catalogs, chemists may spot pyrazinylethanethiol as “2-(Pyrazin-2-yl)ethanethiol,” or sometimes the less technical “pyrazine ethanethiol.” Trade names differ according to region and vendor—names like “Meatthiol,” “FlavorPyrazin,” and “SulthioPyra” try to capture its aroma signature or target customer base. If a food scientist orders it, they’ll usually match the CAS number or IUPAC name, but flavorists working from old notes or company records often pick up a name like “Roasted Meat Note #313.”
Workers dealing with pyrazinylethanethiol learn to treat it with respect right away. Spills fill a lab or factory with sulfurous, eye-watering odor. Skin contact sometimes irritates, and inhalation can tickle the lungs, so basic personal protective equipment—gloves, goggles, lab coats, and proper ventilation—aren’t up for debate. International guidelines like those from OSHA and the European Chemicals Agency set exposure limits to keep health issues off the table. Industry-wide, the focus turns to preventing accidental release, using explosion-proof equipment if handling large quantities. Disposal routes channel the molecule through chemical neutralization tanks or specialized hazardous waste streams.
Pyrazinylethanethiol’s main playground sits in food and beverage flavoring, especially in vegetarian meat analogues, processed meats, soups, and savory snacks aiming for a taste tweak that feels “cooked.” Perfume designers searching for earthy and roasted base notes sometimes sneak it into formulas. Analytical chemists spike samples with tiny quantities to verify detection limits, and animal nutritionists add it to specialty feeds, looking for responses to flavor and palatability. Food safety researchers test its breakdown in cooked or stored foods so that unwanted residues don’t hang around long after the packaging comes off.
Flavor science always looks for new ways to recreate the sense of cooked, roasted, and grilled foods. Pyrazinylethanethiol stands out as a gold-standard molecule for these studies, both as a target to mimic and as a reference point in sensory research. Teams around the globe investigate related molecules, trying to find alternatives with less aggressive safety profiles or that deliver a similar punch at lower concentrations. Some university labs dive deep into the detection thresholds, mapping how human olfactory receptors respond while hoping to make “clean-label” flavors that don’t need exhaustive chemical names. AI-driven ingredient analysis and green chemistry are both influencing newer synthesis processes, nudging the molecule into the digital age.
Practical experience reminds us: less is more. Studies on pyrazinylethanethiol’s acute toxicity note that its strong odor encourages workers to avoid overexposure, yet direct inhalation or skin splashes raise real health concerns. Animal studies show low-to-moderate oral toxicity, but repeat dosing over time can build up, affecting internal organs if concentrations exceed typical flavoring uses. Industry experts keep daily intake recommendations far below problematic levels. Groups such as JECFA and the FDA regularly re-evaluate its safety in food applications, relying on up-to-date toxicological data. In workplaces, continuous monitoring and air filtration systems help keep risk out of daily routines.
Looking ahead, pyrazinylethanethiol won’t be left behind as synthetic biology and sustainable chemistry keep pushing. Modern plant-based foods need a convincing “cooked” aroma to succeed, so demand remains steady. New synthesis routes may sidestep petrochemicals, slashing environmental impact. Scientists want less hazardous analogues that keep the meaty punch but drop the sulfur sharpness, opening the door to broader use in more sensitive products. Automation, digital sensors, and machine learning offer exciting pathways for both detection and formulation work, promising better consistency and discovery of new food-safe aroma molecules inspired by pyrazinylethanethiol’s success story.
Pyrazinylethanethiol doesn’t pop up in daily conversation, but anyone who loves roasted coffee, grilled meats, or groans at the thought of a skunk encounter has likely brushed up against sulfur-containing chemicals like it. This compound grabs attention for its sharp, meaty aroma, and that’s not an accident. Flavor companies bank on its impact. The reason is simple: pyrazinylethanethiol takes bland foods and gives them an irresistible savory kick. In the flavor world, chemists chase after these trace substances because they pack a punch far beyond their quantity.
Industry insiders know pyrazinylethanethiol mainly as a flavor additive. In particular, it shows up in products that need that cooked, roasted note. Take a cheap broth or an instant noodle packet. I once did a side-by-side taste test—plain boiled water with noodles, and the same bowl after adding the seasoning packet. It was night and day. That rich, savory background, almost like there was a slow-simmered stock, comes from trace compounds like this one, designed to mimic what happens in real kitchens.
Coffee stands as another classic example. Even though most folks think caffeine draws them back for cup after cup, the smell and taste play a huge part. After hunting through academic papers and talking to a chemist for an article, I learned pyrazinylethanethiol and similar molecules pop up during roasting as sugars and proteins break down. Food scientists borrow those same reactions when building flavors for vending machine coffee, freeze-dried products, and even pet food.
The food industry prizes this compound because it delivers that “something’s cooking” illusion in places where real meat or roasting isn’t practical. I spent a summer in a test kitchen, and saw just how far producers will go to make plant-based and processed foods desirable. They use a handful of key sulfur-based chemicals, including pyrazinylethanethiol, to create an appealing sense of depth. Without compounds like this, those foods fall flat—think about the cardboard taste of many old-school vegetarian meals.
Beyond food, the compound finds niche roles in fragrance, especially to mimic that roasted, almost nutty smell in certain perfumes. Its strong odor works in minuscule doses, so manufacturers handle it with care. I’ve watched lab techs use pipettes and gloves with these flavor chemicals, because even a speck on skin sticks around for hours.
Pyrazinylethanethiol brings incredible power, but there’s always a line between “delicious” and “what on earth is that smell?” I remember reading accounts from flavorists about customers who complained that some enhanced foods almost smelled like overcooked vegetables or worse. Sulfur is tricky business: too much and it’s off-putting.
Synthetic flavor engineering raises lots of questions about health, authenticity, and transparency. Regulations limit how much can be used, and I’ve seen ingredient lists where it’s tucked under broad terms like “natural flavor.” Some people are skeptical, and for good reason. They want simple food, not a lab’s best guess. Encouraging more open communication between manufacturers and eaters, along with steady research into the safety of such additives, could ease concerns.
At the end of the day, flavors like pyrazinylethanethiol show how chemistry shapes our experience of food and memory. A little goes a long way, making bland snacks craveable or cheap coffee taste like a much richer brew. As someone who’s spent years analyzing ingredient labels and talking with food chemists, it’s clear compounds like this aren’t going away, but finding balance—between creativity and honesty—means everyone wins at the dinner table.
Working in a lab gives you a good sense of what can go wrong with careless chemical storage. Pyrazinylethanethiol, a commonly used flavor compound, can cause problems if left unchecked. It releases a strong, pungent odor—even a tiny amount in the air becomes obvious. Some might joke about the smell, but the reality is a spill can ruin a whole day for everyone nearby. The main issue is more than just discomfort; mishandling introduces health hazards and contaminates surrounding workspaces.
Pyrazinylethanethiol breaks down under sunlight and heat, which doesn’t just mess with its stability but can also change its chemical structure. I’ve seen batches degrade after being left near a window, turning a planned experiment into wasted hours. Chemical breakdown brings in more risk—not just for the person responsible but for those handling the next step, who won’t know the batch has begun to change. Putting storage in a cool, dark space isn’t just about following a rule. It means you know what’s in the container stays the same from one day to the next.
Plastic bottles never worked out for our team. Pyrazinylethanethiol likes to escape soft barriers. Leadership switched to using glass containers with secure caps, and leaks stopped. Less odor, no sticky residue, and fewer complaints. Even in flavor labs, glass troubles me less than plastic—which sometimes lets through those stubborn scents no matter how tight the seal.
Leaving lids open or working near running water invites disaster. This compound reacts in damp conditions, clumping up or changing in ways you don’t want. Once, a colleague left the cap loose, and we all paid for it—stale aroma in the storage cabinet, with the batch ruined. Dry storage, with packets of desiccant, saved us from repeating that problem. A small investment in moisture control goes a long way.
It’s not just the technical side of science that matters. Keeping a clear label on every container avoids accidental misuse. Someone once poured the wrong chemical into a mix simply because two bottles sat near each other and looked similar. Manual labeling, bold and waterproof, turned a risky guessing game into a routine task. Such practical organizing strategies aren’t flashy but often save real money and time.
One whiff of this compound tells you ventilation matters. Fume hoods make a real difference. We use fume hoods every time Pyrazinylethanethiol comes out of storage. Even filling small vials, the fumes build up if left unchecked. Installing and maintaining working ventilation hardware may sound like an overhead, but in reality, it prevents headaches, both literal and figurative. Anyone trying to work in an unventilated space quickly learns this chemical creates a miserable atmosphere—working eyes, sore throats, and a stubborn smell that doesn’t go away.
Cleaning up after using Pyrazinylethanethiol should never lag behind. Sealed waste containers, labeled properly, keep problems from spreading outside the lab. Local regulations often require specific disposal, and these rules exist for good reason. Dumping this compound down the drain endangers more than just immediate coworkers. Paying attention to disposal—like using absorbent materials trapped in tight bags—removes the worry of lingering odors or long-term environmental headaches.
I’ve spent a few afternoons in labs where everything smelled odd and chemicals like pyrazinylethanethiol made their rounds. In some circles, people use it because of its potent flavor and fragrance qualities. It’s the sort of compound that shows up in roasted or cooked flavors—great for food scientists and perfumers who like to create a strong sensation with just a pinch.
The trouble starts with its smell. One whiff and you’ll understand why anyone would ask about its safety. Even tiny amounts make the air feel thick—messing with more than just your nose. My experience tells me that strong odors usually mean you should treat the chemical with respect. Manufacturers handle it inside properly ventilated spaces, wearing full protection. This isn’t just for peace of mind; the stuff can irritate skin, lungs, and eyes in a hurry. I watched a coworker get careless once: even through gloves, the stench lingered for hours, and his hands felt itchy for the rest of the day.
Look through safety data sheets, and you’ll spot warnings about acute toxicity. Pyrazinylethanethiol isn’t the most dangerous chemical around, but breathing it in or letting it touch your skin risks reactions. The short-term problems often include headaches, coughing, and eye irritation, or worse if someone keeps at it. No one is lining up to test what happens over years of exposure, but similar chemicals have caused chronic breathing problems and allergic reactions for people who handle them without proper gear.
Potential long-term impacts stay murky—many flavor ingredients simply haven’t been studied in deep human trials. But folks in the industry keep tabs on their exposure. After all, chemical safety isn’t just about what happens today; it’s also about the risks people take on over a career. That’s something easy to ignore if your main experience comes from reading, not working with the stuff.
Industrial use means tight regulation. In the EU, pyrazinylethanethiol lands on a list where companies must track and report its use. The U.S. complies with similar rules. In my corner of the world, that’s translated into real changes. Places I’ve worked have upgraded ventilation, swapped powders for safer liquids, and started using closed transfer systems so the air stays clean for everyone. It’s cost money, but folks breathe easier—literally and figuratively.
Beyond the workplace, tiny traces end up in finished products—well below levels thought to cause harm. Regulators keep these limits low for a reason: a little goes a long way, both in taste and risk. Whenever companies mess up and higher amounts leak out, recalls follow. It hurts trust and ends up costing more than extra safety measures would have in the first place. If there’s one lesson I’ve learned, it’s that cutting corners with chemical safety always backfires.
Direct experience with day-to-day handling points toward a few fixes. Good ventilation makes a huge difference; you don’t realize how important clean air is until you lose it. Training sits at the core—no one wants to read a stack of safety sheets, but real stories about skin rashes or trips to the nurse make an impression. Personal protection—eye covers, gloves, and chemical-resistant coats—aren’t stylish, but after you see what can happen, you never skip them again.
No one can predict all the risks, but most accidents vanish when companies take safety seriously. Chemical flavor compounds like pyrazinylethanethiol come with trade-offs: bold taste at the cost of handling it with care. Smart businesses never forget that—and neither should the folks who work with these compounds every day.
A lot of people outside chemical or flavor manufacturing circles probably haven’t heard about pyrazinylethanethiol. In reality, a small dose of this compound makes food manufacturing and even some hand-crafted products possible. This compound shows up most in flavors and fragrances, especially where folks are chasing that roasted or grilled aroma which makes snacks addictive even before you taste them. Food chemists and perfumers talk about purity in a way that almost sounds poetic sometimes, but it all comes down to business. The cost, the compliance, and the safety issues start right at the purity level a supplier can guarantee for each batch.
I’ve spent time on the purchasing side of specialty chemicals, and most commercial samples of pyrazinylethanethiol tend to show up with purity claims sitting between 95% and 98%. This isn’t pharmaceutical grade, but it’s above the mark where an unwanted side reaction or a weird off-note ruins an entire day’s work on the production line. If you walk through the food flavor industry, nobody wants a pungent by-product slipping through; even at one percent, contaminants create real headaches.
Digging through supplier datasheets, Sigma-Aldrich and a few specialized companies consistently market the material at these levels — usually with proof from gas chromatography testing. The rest of that two to five percent? Typically, you’ll find closely related sulfur or nitrogen compounds sitting there, which pop up from the inevitable side reactions during synthesis. These small differences in purity numbers change how companies handle storage and delivery. I’ve seen a producer refuse a shipment because it landed at 89%. The spec mattered that day, not just for analytical perfection but because it meant they didn’t have to go through another round of filtration.
Most people outside of industry don’t realize how big of a deal purity is for even one drum of material. High-purity pyrazinylethanethiol brings peace of mind: the scent profile lines up with what the perfumer expects, and regulators breathe easy knowing nothing weird or unexpected ended up in a consumer-facing product. I remember a client who mixed a low-grade thiol into their barbecue chip batch once. The final product was “technically” on spec, but consumer complaints about odd aftertastes hit social media and retail sales slumped for a quarter.
Purity also affects safety. Even food-safe chemicals become a liability with the wrong contaminants. Regulators in Europe and the United States will request not only a Certificate of Analysis but details about each impurity, down to tenths of a percent. Those out-of-spec reports build up fast and can put a supplier on a watch-list for years.
Manufacturers chase reliable methods to boost purity, usually by tweaking synthesis conditions or investing in better purification steps like fractional distillation or advanced chromatography. That pushes prices up, but it means end users can trust their supply. Some supply contracts even insist on independent analysis of every batch, and from experience there’s less hassle and more predictable business once both sides agree on those standards ahead of time.
Smaller producers might sometimes take shortcuts, but as the market matures, more brands partner with labs for batch-to-batch screening. Investing in fast, automated testing helps detect off-spec lots earlier and costs far less than a recall or lost contract. Advances there benefit everyone from the giant food conglomerate to the specialty flavor house in a rented space.
As more trends in food and fragrance chase authenticity and transparency, tighter specs for pyrazinylethanethiol aren’t just regulatory box-checking. It’s quality assurance, it’s risk management, and it’s a real way to build trust with both business partners and end customers. From personal experience, the best-run companies see that purity isn’t a detail; it’s the foundation for everything else they’re building.
Pyrazinylethanethiol carries a punchy odor, landing somewhere between roasted nuts, cooked meat, and earthy undertones. Industries tap into this molecule exactly for that reason; it gives flavorists and fragrance experts a surprisingly powerful tool to mimic grilled favorites, coffee, or to boost savory notes that keep customers coming back. Any company blending flavors for snacks, plant-based meats, or specialty seasonings wants to get their hands on stable, high-quality supply — and not just a few grams at a time.
Once you leave the lab-scale catalogs, roadblocks pop up. Ten grams here or twenty grams there, fine for R&D, but that doesn’t go far in a factory churning out soup bases or protein bars. I tried sourcing this compound for a flavor project a while ago and hit a wall; vendors treat pyrazinylethanethiol almost like a rare spice, not a bulk commodity. Most bulk ingredient suppliers skip it entirely, often due to how tricky its synthesis can get and the stink it leaves behind during shipping and storage.
Production usually stays limited because the synthetic process gets complicated — intermediates don’t always cooperate, and nasty odors can escape even double-bagged containers. This keeps the price higher than vanilla or citral, driving away buyers unless they really need the edge pyrazinylethanethiol delivers.
Some suppliers take orders but drag out timelines, hunting for time in their schedule between other, less troublesome syntheses. Regulations also cloud things. Industrial buyers sometimes wave a “hazard” label if they spot high volatility or environmental risks. Pyrazinylethanethiol, with its sulfur notes and potential for workplace smells, nudges suppliers to think twice about ramping up production without a guaranteed buyer. Factory neighbors don’t soon forget a leak of sulfurous aroma inside city limits.
Talking to industry friends, most say demand hasn’t hit that tipping point where suppliers overhaul infrastructure or strike deals with logistics firms ready to handle stinky freight. Some European and Japanese suppliers flex their chemistry skills and do occasional bulk runs, but costs stay steep and minimum order sizes make smaller companies shy away.
If the plant-based boom and complex snack markets keep growing, the squeeze for bulk quantities of tuneable aroma chemicals will only tighten. Industry moves can help. Cooperative purchasing between smaller companies can drive up order volumes and push suppliers to consider larger synthesis batches. Experienced transporters — those moving animal feed additives, for example — already have systems to contain and minimize sulfur smells, making them well-suited for these tasks.
On the supply side, payoffs come with investment in better process controls and purification, letting suppliers crank out cleaner product with less waste. Universities and contract research groups could find easier or more environmentally friendly synthesis routes, slicing costs. Regulatory clarity, with guidelines for safe handling and shipping, might coax new players into the market.
For now, demand still outweighs accessible supply. Anyone needing lots of pyrazinylethanethiol will have to hustle — and probably budget a little more time and cash for the privilege.
| Names | |
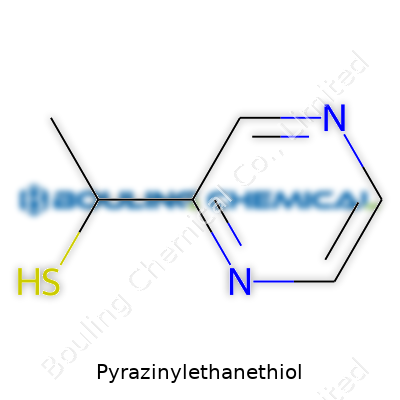
| Preferred IUPAC name | 2-(Pyrazin-2-yl)ethane-1-thiol |
| Other names |
2-(Pyrazin-2-yl)ethanethiol 2-(2-Pyrazinyl)ethanethiol |
| Pronunciation | /paɪˌræzɪnˌaɪlˈɛθəˌnaɪˌθaɪɒl/ |
| Identifiers | |
| CAS Number | [13337-18-7] |
| Beilstein Reference | 5565175 |
| ChEBI | CHEBI:38768 |
| ChEMBL | CHEMBL224093 |
| ChemSpider | 142047 |
| DrugBank | DB08226 |
| ECHA InfoCard | 08f14ea9-8809-4b63-96e5-f2e4257211d5 |
| EC Number | EC 284-996-8 |
| Gmelin Reference | 595867 |
| KEGG | C18622 |
| MeSH | D000077328 |
| PubChem CID | 137328130 |
| RTECS number | UF8225000 |
| UNII | WZ8M0Q2388 |
| UN number | UN3335 |
| Properties | |
| Chemical formula | C6H8N2S |
| Molar mass | 142.22 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | sulfurous, onion, roasted, meaty |
| Density | 1.199 g/mL |
| Solubility in water | Insoluble |
| log P | 1.030 |
| Vapor pressure | 3.62E-02 mm Hg at 25°C |
| Acidity (pKa) | 7.7 |
| Basicity (pKb) | 1.86 |
| Magnetic susceptibility (χ) | -77.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.624 |
| Dipole moment | 3.47 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 322.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -636 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS06, GHS07, GHS08 |
| Pictograms | GHS05,GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H301, H311, H331, H373 |
| Precautionary statements | P261, P280, P304+P340, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-3-2 |
| Flash point | 82 °C |
| Lethal dose or concentration | LD50 (oral, rat): 246 mg/kg |
| LD50 (median dose) | LD50 56 mg/kg (rat, oral) |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.003 ppm |
| Related compounds | |
| Related compounds |
Methanethiol Ethanethiol Pyrazine Pyrazinylmethanethiol |