Stories in science rarely begin with a bang, and pyrazine proves that well. First isolated and named by German chemists in the late 19th century, pyrazine made its quiet debut as a curious byproduct of coal tar distillation. In early labs, researchers puzzled over its pungent odor and bitter taste, documenting how its crystalline structure stood apart from the simpler building blocks they understood. As organic chemistry evolved, so did interest in this six-membered nitrogen ring. By the mid-1900s, as synthetic chemistry kicked into high gear, scientists started pinpointing pyrazine occurring naturally in roasted coffee, seeds, even grilled meats. It became clear—pyrazine showed up in flavors people already craved and scents that sent signals across both fields and laboratories.
In its pure form, pyrazine carries an earthy, somewhat nutty aroma and a sharp taste that some folks describe as "burnt" or "toasty". You’ll spot it in vials labeled for use in making flavors, fragrances, and as a chemical intermediate. Industrial producers isolate and sell both the basic molecule and a family of modified versions—methyl-, ethyl-, and other alkyl-pyrazines—each with its own signature punch. Whether blended into food flavoring, packed into fragrance supplies, or delivered to chemical plants, pyrazine never stands still long in one industry.
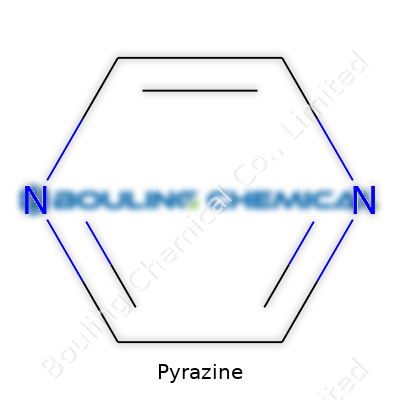
Look up pyrazine in a handbook and you’ll see a white to yellowish solid, melting at 52-55°C and boiling just above 115°C. It’s got low solubility in water but mixes with most organic solvents. Every chemist knows its formula: C4H4N2. Two nitrogen atoms on opposite sides of the benzene-like ring change everything; pyrazine reacts differently from its all-carbon cousin. In air, it takes on moisture, which makes handling and storage a bit particular. Pyrazines remain stable under most lab conditions, resisting breakdown until heated strongly or hit with reactive agents.
At industrial scale, suppliers set benchmarks for content, purity, and trace contamination. Labels on drums will feature CAS number 290-37-9, plus warnings about flammability and inhalation risks. Material data sheets call for 98% or better assay, list melting and boiling points, and include required regulatory notations for workplace safety. Clear specification on batch, lot, and shelf-life rules out mix-ups. In my own time handling chemicals, nothing brings more peace of mind than seeing full compliance straight on the canister.
These days, most commercial pyrazine comes from controlled reactions between glyoxal and ammonia. The process kicks off in stainless reactors under snug temperature controls, with reaction time and pressure closely monitored to avoid unwanted byproducts. After formation, the mixture cools, and then out comes pyrazine in crude form, usually separated by distillation. Purification often takes several re-crystallization steps, stripping away impurities that might give the end-product a chemical tang, rather than the clean, crisp note customers want. Lab-scale routes usually mimic this industrial method, only with smaller vessels and more hands-on tinkering.
Pyrazine loves to play host to all sorts of chemical guests. Replace one hydrogen with a methyl or ethyl group, and the smell drifts toward roasted nuts or popcorn. Add a complex side-chain and new flavors emerge, sometimes rich, sometimes sharp or musty. Synthetic routes open doors to derivatives with pharmaceutical punch—think anti-tumor, anti-microbial candidates. Chemists often subject pyrazine to acylation, alkylation, or even more ambitious coupling reactions to build libraries of new molecules. Each twist brings a fresh possibility for use, and good labs keep up by cataloguing reactivity and outcome for every new spin on the ring.
Pyrazine carries a few aliases: 1,4-diazabenzene, p-diazine, and in flavor circles, sometimes just “toasted note.” Commercial bottles might carry brand names tacked on—PyraFlav, NutraPyra, and others my industry contacts would instantly recognize. For regulatory filing, sticking to “pyrazine” keeps paperwork tight and avoids run-ins with customs or compliance officers. In R&D circles, scientists get precise and specify which pyrazine derivative sits in the test tube: 2-methylpyrazine, 2,5-dimethylpyrazine, etc.
Every workplace that runs pyrazine works under strict codes. Storage means vented containers in cool rooms; the molecule won’t ignite easily, but fine dust or vapor can still catch with the right spark. Gloves, goggles, and a face mask aren’t overkill—pyrazine fumes dry out mucous membranes and lingering dust can irritate skin. Material safety data guides spell out spill management, emergency wash procedures, and vapour control. In my experience, training for new staff always includes the MSDS, and a drill on safe handling. Disposal through an approved chemical waste stream prevents environmental hiccups, and audits on labeling and storage spare everyone from last-minute panic.
Most folks brush up against pyrazine in food and fragrance. Its own nutty, earthy taste brings depth to artificial flavoring for snacks, coffee, and even tobacco blends. Confectioners use it for pralines or roasted nut profiles; beverage makers slip it into drinks for added complexity. In perfumery, subtle amounts carry that signature “roasted” lift. Labs reach for pyrazine building blocks to craft new medicines, leveraging the ring’s chemistry for anti-fungal, anti-viral, or anti-tumor leads. Agricultural scientists test derivatives as potential pesticides and plant stimulants. Across so many industries, pyrazine rarely sits still: its applications grow as R&D teams explore chemical tweaks and field trials.
R&D in the pyrazine world rarely slows. Universities and private labs run screens for novel biological activities; big pharma hunts for new therapeutic options built around the sturdy diazine ring. Synthetic chemists constantly devise new routes for making pyrazine and its countless cousins at higher yields, lower cost, and with less environmental baggage. Analytical teams fine-tune detection methods, examining traces in food, water, and environmental samples to understand exposure — a step up from the cruder tests I remember from my own early lab days. Equipment upgrades, automation, and digital tracking boost throughput, speeding up how fast molecules travel from idea to application.
Toxicologists keep a sharp eye on pyrazine’s safety in food and workplace air. In animal studies, only high doses cause trouble, and typical exposure in food or fragrance falls far below regulatory limits. Skin tests report mild irritation if liquid pyrazine stays too long on exposed arms, and inhalation of concentrated fumes brings cough and sore throat. Some long-term studies check for carcinogenic effects but find no strong evidence at everyday exposure levels. Government watchdogs like the FDA and EFSA monitor new research and adjust “safe level” guidance as more data comes in, a process I trust for peace of mind on the job and at the grocery store.
Down the line, pyrazine’s story likely grows even bigger. Novel synthetics promise bolder, safer, and more sustainable food flavors that edge out costly natural ingredients. Pharma pipelines probe the ring’s many points for drug activity, chasing treatments for infections, neurological disorders, and more. Green chemistry initiatives target cleaner, renewable starting materials, challenging industry to swap petrochemicals for bio-based sources. Environmental monitoring sharpens focus on trace contamination, pressing labs to detect ever smaller residues in soil and water. As research deepens, pyrazine’s legacy shifts—from coal tar’s odd cousin to a cornerstone of modern living, one molecule at a time.
Pyrazine might sound like something locked away in a chemistry lab, but the truth brings it far closer to our daily lives than most of us realize. Anyone who enjoys the flavors and aromas of coffee, roasted nuts, or even a slice of bread just out of the oven has encountered pyrazine, even if unknowingly. This compound helps create the nutty, earthy, chocolatey notes people cherish in their food. Pyrazine sneaks onto the ingredient lists of everything from snack foods to drinks. Its ability to mimic roasted, toasted, and peppery flavors lets food companies intensify those flavors without needing to go through slow, costly roasting for everything. That’s why pyrazine has become a go-to flavor booster for manufacturers chasing consistency and shelf appeal.
In my early college days, I thought artificial flavoring was pure trickery—just chemicals pretending to be the real thing. After a summer internship at a small snack factory, my perspective flipped. Pyrazine-based flavorings solved real problems. Roasting hundreds of kilos of peanuts left some sweet, some bland, some bitter. With a dash of pyrazine, the “nutty” came through every single time. One batch tasted like the last, and the savings on raw materials and energy were huge. This mattered for small companies trying to keep prices steady, especially as costs climbed.
Food safety matters just as much as flavor. The FDA keeps pyrazine under control, labeling quantities safe to eat. Research out of the European Food Safety Authority shows that the levels used in food don’t present health, toxicity, or allergy risks for most people. Mistakes can happen with unregulated concentrations, but oversight and strict recipes are now part of the industry’s best practices.
Taste isn’t the only playground for pyrazine. The medicine cabinet sees its fair share of this compound. Drug researchers mix pyrazine into some cancer treatments and nervous system medicines. Its ring shape, easy for clever chemists to modify, makes pyrazine a flexible starting point for designing molecules that home in on the body’s tricky targets. Some anti-tuberculosis drugs carry the mark of pyrazine, providing a backbone for antibiotics still working where others have failed.
Pyrazine’s power to imitate comforting smells has also found a calling in perfumes and air fresheners. Perfumers layer it in when building “woody” or “nutty” fragrances that need stronger backbone. These scents weave their way into homes and clothes, an invisible presence thanks to a molecule few people outside science know.
There are stories about people getting queasy from strong food flavorings, or headaches after a whiff of a new air freshener. Most of the time, these effects trace back to doses far above recommended limits or some quirky personal sensitivity. My aunt avoids processed cheese altogether since the “fake” cheese flavors put her off. But stepping away from processed foods has benefits that reach far beyond just avoiding flavor additives—especially for folks trying to eat less salt, sugar, or fat.
Environmental safety doesn’t get talked about as much as it should. Factories can dump pyrazine byproducts into water and air unless they stick to modern pollution controls. Communities living near old industrial facilities sometimes face water contamination. If stronger rules forced companies to update equipment and clean up, people wouldn’t have to worry so much about side effects outside the kitchen or medicine cabinet.
A quick fix isn’t always the right answer, even for food companies or pharmaceutical labs. Using pyrazine responsibly means keeping recipes honest and always looking for safer, more natural ways to deliver flavor or medical benefits. Individual cooks and home gardeners rediscovering old roasting and fermenting methods remind everyone that real flavor builds up slow—but lasts a lifetime. Industry would be smart to take that lesson to heart, remembering that convenience and authenticity don’t always need to clash.
Pyrazine shows up in all sorts of flavors, mostly in the roasted, nutty, or popcorn category. If you’ve ever smelled a fresh cup of coffee, hot bread, or the golden outside of a batch of roasted peanuts, you’ve bumped into this compound. Food companies add it because it boosts the aroma, making simple snacks taste like they’ve been cooked over a fire or baked from scratch. It’s a tiny thing that punches way above its weight in terms of flavor.
Folks working behind the scenes in food factories aim for the kind of flavor that makes people want another bite. Natural pyrazines pop up during cooking, but to keep costs down and provide consistency, labs have figured out how to make versions for mass production. This raises questions from curious eaters who want to know if something made in a lab is okay to eat.
Governments care about what we put in our bodies. Both in the US and Europe, food safety authorities have already spent time on pyrazine. The FDA in the United States has put several types of pyrazines on their safe-to-eat list, assuming you don’t eat truly wild amounts. The European Food Safety Authority also checked the evidence and ended up in the same spot — safe in the tiny quantities you’ll find in popcorn or candy.
No food chemical gets a lifelong free pass. Regulators take new research seriously and update rules if red flags pop up. But for pyrazines, the well-conducted animal studies haven’t shown problems unless scientists pumped animals up with huge doses, way past what a person could eat without making themselves sick from the food alone.
Much of what makes people nervous about these additives has less to do with the stuff itself and more to do with the amount, the context, or suspicious headlines. The math says that the average person swallows way less pyrazine than doses scientists test in toxicology labs. Instead of shoveling spoonfuls of pure flavor compound, most people taste pyrazine by the microgram, often spread across an entire bag of chips or puff of cereal.
It’s fair for people to wonder what’s hiding in their food, and skepticism has value. But flavor scientists and food safety teams spend years tracking how small shifts in consumption might tip the scales. They look at not just a single snack, but how much a population consumes over a lifetime. Consistent findings for pyrazine reassure both regulators and dietitians — nobody’s shown harm at the levels we typically see.
Plenty of folks struggle with food allergies, but pyrazine isn’t triggering those types of reactions. Instead, most natural foods like nuts cause trouble because of proteins, not small molecules like pyrazines. Some concerns pop up about how these flavor chemicals blend with other additives, but nothing in the evidence vault hints at some hidden risk from eating pyrazine-flavored foods on a daily basis.
Too much of anything throws the body off, but pyrazine’s not unique in that way. The real headache happens with overeating processed food in general — all the salt, sugar, and fat that hide in the mix alongside savory flavor deals most of the health damage. Focusing on overall diet quality matters more than singling out this one compound.
Companies and regulators do a decent job watching pyrazine’s safety, but transparency still needs work. Publishing clear, everyday-language safety reports builds trust. Food labels could do more than just list flavor as a vague term. If a snack uses pyrazines, why not say so? Real food understanding grows when everyone knows what’s going into their lunchbox or coffee mug.
At the end of the day, most questions about processed food safety won’t go away. Pay attention to credible research and balanced diets, and most single flavor compounds look a lot less scary than the headlines would have you believe.
Anyone who’s opened a bag of roasted coffee knows that bold, slightly nutty smell. Much of that magic traces back to pyrazine compounds. Food manufacturers reach for pyrazines because even tiny amounts create deep, toasty notes that mimic roasting or baking. Corn chips, grilled meats, cocoa—these wouldn’t taste as authentic without a touch of pyrazine. Snack producers learn to balance pyrazines for maximal appeal, because people recognize “natural roasted” flavor even if they’ve never set foot in a smokehouse. The biggest brands don’t shy away from these chemicals either, because marketplace taste tests nearly always pick the snack with the rounder, richer, pyrazine-backed flavor.
Beyond taste, pyrazines pop up in perfumes and personal care products. Perfume houses use them to add warmth and realism. A whiff of earth, nuts, or sweet bread brings comfort, and perfumers find that pyrazine delivers those grounded notes even in a bottle.
Farmers face crazy amounts of pressure from insects and plant diseases. Crop protection companies add pyrazine compounds to pesticides because bugs dislike—or just can’t eat—leaves coated with them. Pyrazines create strong odors or tastes that make certain insects leave crops alone, while some synthetic blends can trick bugs entirely, leading them away from growing acres of food. This can mean farmers use less spray, saving money and protecting pollinators.
Beyond pest management, research in plant biochemistry leans on pyrazines, too. Some studies explore their role in helping plants withstand stress. It’s not often headline-grabbing science, but every step forward helps keep food affordable and reliable.
Drug makers value small molecules that can fit specific “pockets” in the body’s proteins. Pyrazine isn’t just a pretty flavor; it’s a starter block chemists use to build new medicines. Dozens of antibiotics and cancer treatments wouldn’t exist without it. Take the tuberculosis drug pyrazinamide, a staple in fighting a disease that still haunts millions worldwide. Medicinal chemists tweak pyrazine rings to change how substances interact with skin, blood, or brain, pushing forward the next generation of treatments.
Working in pharma taught me that chemists look for stable, low-reactivity scaffolds—something pyrazine delivers—then build complexity. Putting the right functional group on a pyrazine ring shifts its activity, with outcomes ranging from life-saving medicines to targeted antivirals. Its reliability as a molecular core saves time during development, keeps costs manageable, and offers a leg up against drug-resistant infections.
I’ve seen battery developers use pyrazine as an electrolyte component. The search for safer, more powerful batteries for electric vehicles and handheld devices uncovers surprising uses for compounds that started in flavor labs.
On the specialty chemicals front, pyrazine often goes into dyes, resins, and corrosion inhibitors. These applications may not earn prime-time attention, but their absence would leave manufacturers in a pinch. Pyrazine helps paints stick better and last longer on ships, bridges, or even your backyard grill.
Every industry looking for greener processes gives compounds like pyrazine a fresh look. As researchers keep baking up new uses, this once-niche chemical keeps popping up in places I wouldn’t have guessed a decade ago.
Pyrazine sounds like a complicated science word, but most people have tasted it—smelled it, too. Coffee roasting brings out its nutty scent. Fancy food scientists toss it around when explaining why potato chips taste the way they do. The perfume industry dabbles with pyrazines to make some scents richer and earthier. But with so much use in food and fragrance, it’s worth asking what this stuff really does to the body.
Eating or smelling pyrazine in everyday doses doesn’t send most people running to the emergency room. Scientists haven’t found it builds up in the body over time. Reading through journals and food safety reports, I find nothing about pyrazine itself setting off alarm bells at everyday exposure levels. Regulatory agencies like the FDA let it stick around in flavor blends since they haven’t linked it to major health risks in the form and amounts used for foods.
Like a lot of chemicals, dosage matters. Swallow enough water and it turns dangerous. Pyrazine follows the same idea. Studies with lab animals, often at doses way above what you’d get from your lunch, mention headaches, dizziness, breathing discomfort, or a burning sensation. People working in factories making tons of flavor compounds—breathing pure vapors, getting high concentrations—face more risks. I can picture a technician in a small lab, huffing strong fumes without proper gear, dealing with nose or throat irritation or feeling light-headed. The average person munching chocolate with a sprinkle of pyrazine flavor hardly faces the same concerns.
Pyrazine isn’t just one thing—it’s a family of compounds. Some versions, like methylpyrazine, show up often in roasted food and have a decent safety record. Others act rougher on the body at lower hits. Odd flavors that smell burnt or chemical at high concentrations signal a natural stop sign. Our noses don't usually like things that hurt us.
People who spend decades in food manufacturing might deal with accidental spills or leaks. I remember a fellow worker, once splashed by a chemical mixture in the flavor lab, running for the eyewash station. Even though it wasn’t pure pyrazine, it gave me a sense of how harsh concentrated chemicals feel on skin or eyes. Direct contact at high strengths means irritation, sometimes worse. At home or sitting in a café, people rarely meet these extremes. Still, it pays to keep flavor and fragrance mixing where people know how to protect their lungs and skin.
Real risk comes with high concentrations and direct exposure. In food, tiny flavor doses get approval for a reason. Most risks fade away for the average person sharing popcorn at a movie night. Still, it helps to keep an eye on what gets added to foods, especially with new synthetic flavor molecules. Good labeling, workplace safety standards, and a bit of caution go a long way. As the world craves new tastes and scents, making sure safety rules stay strong helps keep pyrazine a friend, not a foe.
Pyrazine often shows up in flavors, fragrances, and even pharmaceuticals. It carries a nutty, roasted smell that you might recognize, though few know it by name outside the lab or factory floor. Anyone dealing with this substance—be it a small food company or a big chemical plant—must know that it can be tricky if treated casually. Pyrazine comes in a solid or liquid form, but both share one thing: they deserve care, not just a spot on a dusty shelf.
Pyrazine has its quirks. It’s flammable. In an enclosed, unventilated space, vapors can build up and lead to dangerous conditions if there’s any spark or open flame. It might not be explosive by itself, but no one wants to test their luck. Breathing in those fumes brings on health concerns—irritation in the eyes, skin, or even problems if enough is inhaled. I remember chatting with a colleague years ago who underestimated this compound and wound up with headaches and irritation after letting a bottle stay open in a cramped workspace. We all learned from that day to not treat pyrazine as just another bottle among hundreds.
Let’s talk real-world measures. Keep pyrazine in tightly closed containers—think glass, high-quality plastic, or metal that won’t react with the chemical itself. Ventilation comes before comfort. Open jars or flasks only in areas where fresh air moves freely, or better yet, stick to chemical fume hoods if possible. Store away from direct sunlight, high heat, or ignition sources. No one wants an accidental fire in the middle of a busy afternoon.
My own lab keeps flammable liquids and solids in designated safety cabinets, and pyrazine belongs right in there, away from strong acids or oxidizers. Segregation isn’t just a bureaucratic rule—it’s the difference between a typical workday and an emergency that sends everyone running for the exits.
Personal protective equipment isn’t just for show. Gloves made from nitrile, snug-fitting safety goggles, and long sleeves close off easy entry points for fumes or spills. Even the most careful operator can fumble on a rough Monday morning, so always plan as if the worst could happen.
Disposal matters as much as storage. Pyrazine should never get poured down the drain or tossed in the regular trash. Work with hazardous waste handlers who know the ropes and can take it off your hands safely. Regulations may vary, but it pays to lean on professionals with experience.
In my experience, mistakes don’t happen from ignorance—most slip-ups come from familiarity. If everyone is trained with real examples, not just a list of rules, the workplace feels safer. Share stories about close calls, troubleshoot procedures as a team, and check the chemical labels with fresh eyes from time to time.
I’ve seen enough in the field to say that safety with pyrazine isn’t about bureaucracy; it’s about respecting the risks and respecting the people who work near it. Every bottle on a shelf tells a story. Know yours, and treat it with the attention it deserves.
| Names | |
| Preferred IUPAC name | Pyrazine |
| Other names |
1,4-Diazine Piperazine Tetrazine Pyrazin |
| Pronunciation | /ˈpaɪrəˌziːn/ |
| Identifiers | |
| CAS Number | 290-37-9 |
| Beilstein Reference | 605675 |
| ChEBI | CHEBI:35571 |
| ChEMBL | CHEMBL18561 |
| ChemSpider | 121 |
| DrugBank | DB03194 |
| ECHA InfoCard | 100.011.760 |
| EC Number | 2.7.1.157 |
| Gmelin Reference | 60738 |
| KEGG | C06141 |
| MeSH | D011717 |
| PubChem CID | 1049 |
| RTECS number | UY8400000 |
| UNII | 2JX945CO2Z |
| UN number | 1323 |
| Properties | |
| Chemical formula | C4H4N2 |
| Molar mass | 80.10 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | nutty |
| Density | 1.03 g/mL at 25 °C (lit.) |
| Solubility in water | soluble |
| log P | 0.65 |
| Vapor pressure | 3.79 mmHg (at 25 °C) |
| Acidity (pKa) | 0.6 |
| Basicity (pKb) | 0.79 |
| Magnetic susceptibility (χ) | -47.6·10⁻⁶ |
| Refractive index (nD) | 1.503 |
| Viscosity | 0.991 cP (20 °C) |
| Dipole moment | 0.00 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | S°₍₂₉₈₎ = 274.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 69.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2637 kJ/mol |
| Pharmacology | |
| ATC code | N05AX08 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation |
| Precautionary statements | P261, P280, P304+P340, P312, P370+P378 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 38 °C |
| Autoignition temperature | 570 °C |
| Explosive limits | Explosive limits: 5.4–21.6% |
| Lethal dose or concentration | LD50 (oral, rat): 970 mg/kg |
| LD50 (median dose) | LD50 (median dose): 420 mg/kg (oral, rat) |
| NIOSH | SN4725000 |
| PEL (Permissible) | 1 ppm |
| REL (Recommended) | 0.1 mg/m³ |
| IDLH (Immediate danger) | 100 mg/m3 |
| Related compounds | |
| Related compounds |
Pyridazine Pyrimidine Pyrazole Quinoxaline |