Pyrazine-2-Carboxylic Acid has sparkled curiosity among chemists for decades. Early research featured the aromatic pyrazine scaffold in the backdrop of heterocyclic chemistry, dating back to when simple organic molecules began supporting drug discovery. Back then, synthesizing stable pyrazine derivatives involved tedious routes and offered limited yields. As analytical instruments improved, chemists nailed down more reliable reactions and better ways to monitor purity. Over time, people recognized this compound as more than an academic exercise, and it transitioned from small-scale workbench projects to pilot plant syntheses, making appearances in both research and industry.
This molecule, recognized under several names such as 2-pyrazinecarboxylic acid and 2-carboxypyrazine, is a pale, crystalline solid with a tangy, faintly sweet scent that hints at its heritage from the broader pyrazine family. Labs and factories rely on its reliable physical form and solubility characteristics, which enable formulation into solutions or other preparations. It holds a place as a reference standard and an intermediate because of the rigid aromatic ring and the single carboxyl substituent. At the bench, researchers know that it comes as a fine powder that clumps under moisture, and so handle it with care to avoid losses and ensure proper storage.
Pure pyrazine-2-carboxylic acid appears white to off-white, showing stability under ambient temperatures, though it resists dissolution in nonpolar solvents. The melting point measures between 235–238°C, so the compound holds its shape during moderate heating. The carboxyl group mildly increases its hydrophilicity, while the pyrazine ring imparts some resilience to acids and bases. People have tested its stability in light and air, and often find it resists breakdown unless subjected to extremes. Solubility data highlight water as a preferred solvent for reactions or formulation work, but solubility rises in basic solutions where its acidic proton can be abstracted.
Suppliers often tag this compound as analytical, reagent, or pharmaceutical grade, offering purity above 98%. Packaging involves amber glass or high-density polyethylene containers that shield from moisture and light infiltration, since degradation jeopardizes both shelf life and downstream applications. Lot numbers and certificate of analysis typically accompany shipments, verifying properties such as melting point, mass spectrometric profile, and elemental analysis. Safety labeling includes chemical hazard pictograms and suggestions for the right gloves and eye protection. The importance of tightly sealed storage—away from strong oxidizers and reducing agents—cannot be overstated for those hoping to maintain a reliable inventory.
Industrial synthesis often employs controlled oxidation of 2-methylpyrazine using strong oxidizers like potassium permanganate or chromic acid. These reactions run under carefully regulated conditions, as over-oxidation or poor workup can slash yields or generate byproducts. After the initial conversion, aqueous workups or an acidification step precipitate the acid, and crystallization follows, yielding the pure product. Conversations with colleagues in process chemistry point out how refining the isolation and purification steps dramatically increases efficiency and lowers production costs. In research labs, microscale adaptations reduce waste and allow rapid screening of pyrazine backbone modifications.
This carboxylic acid, thanks to its position on the ring, stands ready for amide bond formation or esterification, paving the way for custom derivative synthesis in medicinal chemistry projects. Some teams use coupling agents like EDC or DCC to join it with amines, building libraries of new molecules with potential biological activity. The pyrazine core, thanks to electron-withdrawing nitrogen atoms, interacts with electrophiles and can survive modest reductions. Modern researchers functionalize the ring at the 3-, 5-, or 6-position to explore structure-activity relationships, using palladium-catalyzed cross-coupling or metalation followed by quenching. In the classroom or lab, these reactions help students and scientists deepen their understanding of organic transformation logic.
Chemists may encounter this compound listed as pyrazine carboxylic acid, 2-carboxypyrazine, PZCA, or pyrazine-2-carboxylate in different catalogs. Regulatory databases might reflect variance in spelling or format, yet the underlying structure remains unchanged. The presence of multiple synonyms reflects the breadth of industries and geographies employing the molecule, from pharmaceuticals and specialty chemicals to agricultural research.
Ring-fused heterocycles like pyrazine-2-carboxylic acid rarely provoke severe toxicity issues at common exposure levels, though researchers wear gloves and protective eyewear to avoid irritation. Reports mention mild eye or respiratory discomfort upon accidental dust exposure, but appropriate engineering controls such as fume hoods minimize risks. Proper disposal procedures emphasize neutralization with sodium bicarbonate, collection of residues, and treatment as hazardous waste. Regular refresher courses for staff handling chemicals play a major role in reducing workplace incidents; institutions enforce training and audit logs that reinforce this culture of safety.
Medicinal chemistry drives much of the demand for this compound, using its backbone to develop antimicrobials, tuberculostatics, and enzyme inhibitors. Screenings in vitro often test a wide range of pyrazine-2-carboxylic acid analogs for inhibitory activity against target enzymes, particularly since its ring structure commonly fits into biological active sites. Outside human therapeutics, agrochemical research investigates its derivatives for plant growth regulation or fungicidal activity. Analytical labs keep it handy for use as a calibration standard or building block, underscoring its flexibility across industries. Watching peers adapt the molecule for new endpoints reveals how broad research horizons expand even from such a small molecule.
Every year, new publications probe the properties and potential of this compound. Teams create analogs with modified side chains, adding fluorine atoms, methyl groups, or shifting substituents to examine effects on bioactivity. Advances in computational modeling now guide much of this work, streamlining the design phase before anyone runs a single reaction. Researchers share crystal structures, pharmacokinetics data, and potential mechanisms through conferences and journals, building collective knowledge. As demands for green chemistry rise, efforts focus on more efficient catalytic routes and solvent reduction, inspired by both economic and environmental drivers. Collaborations between universities, startups, and established industry labs accelerate the pace, allowing for earlier identification of promising new applications.
Toxicological profiling matters for any chemical—there’s no shortcut on this. Industry and academic labs run standardized assays tracking acute and chronic effects, relying on data from both animal studies and in vitro screens. At moderate doses, results often support low toxicity, though long-term studies on metabolites and degradation products still move forward. Environmental fate analysis monitors breakdown in soil or water, helping regulators gauge risk and suggesting remediation protocols if needed. The requirement for rigorous documentation and transparent data sharing aligns with both public safety and compliance with evolving chemical safety regulations.
The story of pyrazine-2-carboxylic acid looks far from finished. Modern synthetic methods, already more efficient than in the past, appear poised to improve as flow chemistry and enzyme-mediated routes offer cleaner alternatives. AI-guided molecule design signals a boon for rapid analog screening and property prediction, suggesting that the next breakthroughs could come from mapping subtle changes in the molecule's shape. As antibiotic resistance and crop protection demands climb, research targets both classic and unexpected applications, pressing for molecules with precise activity profiles. With greener manufacturing and an expanded toolbox of synthetic techniques, pyrazine-2-carboxylic acid stands likely to feature in both new product development and academic curiosity for a long time.
Pyrazine-2-carboxylic acid might sound like something straight out of a research lab, but it has more to offer than just a complicated name. In my work connecting with professionals in pharmaceuticals and industry, I’ve seen firsthand how such niche chemicals quietly support breakthroughs that shape healthcare and science.
Most folks don’t think about tuberculosis treatments during their daily commute, yet this disease still affects millions worldwide. Pyrazine-2-carboxylic acid plays a backbone role here. It’s a key metabolite of pyrazinamide, one of the frontline drugs used against tuberculosis. Researchers use it to measure drug effectiveness and resistance patterns in Mycobacterium tuberculosis. Reliable TB treatment matters, especially in places where access to healthcare runs thin. Just last year, researchers in India highlighted how measuring this chemical helps spot resistant strains faster. The impact can mean earlier interventions and fewer lives lost.
Curiosity takes pyrazine-2-carboxylic acid into new territories. In labs where folks spend late nights testing catalysts and looking for greener ways to make things, this compound sometimes acts as a building block. Organic chemists find value in its structure when piecing together new molecules for advanced materials, dyes, and agrochemicals. In these cases, a single tweak using a chemical like this can set off a chain reaction leading to new products.
Labs rely on precise measurements, and standards keep researchers honest. Pyrazine-2-carboxylic acid often appears in these quality control routines, making sure results match up across different equipment or between teams on opposite sides of the world. If measurements drift, findings can get thrown off, so tight processes and standards matter more than most folks realize.
No one picks up chemicals in the lab as casually as grabbing salt from the kitchen. Chemical safety training isn’t just a formality—pyrazine-2-carboxylic acid reminds us why. Eating, touching, or even breathing in the dust on accident can lead to health risks. Even though this compound helps fight disease, it comes with its own danger signs. Manufacturers pass along clear protocols to anyone handling, storing, or transporting it.
Chemical production often raises big questions about environmental impacts. I met a chemical engineer last year who described efforts by their company to limit waste and hazardous by-products. Companies producing pyrazine-2-carboxylic acid increasingly look for solvents and methods that trim down pollution while keeping prices reasonable for researchers and hospitals.
Access and cost remain hurdles, especially in lower-income countries where research budgets rarely stretch far enough. Streamlining supply chains and sharing best practices through scientific networks could bring down barriers.
What surprised me most in my time following scientific innovation is how compounds like pyrazine-2-carboxylic acid don't just sit in storage. They spark new treatments, support basic research, and raise the standard for safety and environmental stewardship. As chemical science keeps moving, these unsung players deserve some attention for the practical bridges they build between discovery and solving real-world problems.
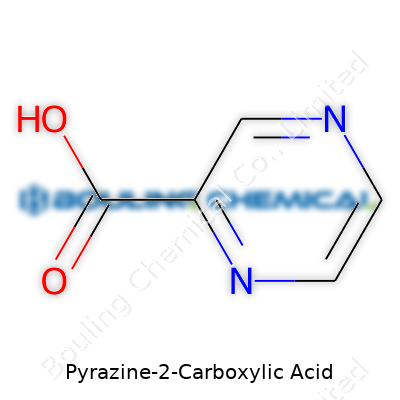
Pyrazine-2-carboxylic acid, carrying the formula C5H4N2O2, brings together five carbon atoms, four hydrogens, two nitrogens, and two oxygens. This structure tells a clear story: a pyrazine ring, stitched with a carboxylic acid group, not just a simple aromatic but one often discussed among chemists for its interesting binding and reactivity. You see similar building blocks in molecules with active biological roles, which turns a curiosity into something practical.
The molecular weight lands at 124.10 g/mol. Anyone working in a lab will spot this figure and start running calculations. Let’s say you plan on using the acid as a starting material. That number tells you exactly how much to weigh out for a reaction. Beyond the basics of measurement, this value shapes how the compound travels in a liquid or passes through a column during purification. For pharmaceutical research, weight calculations guide dosage and stability research.
This molecule pops up in research papers focused on antibiotics. Pyrazine-2-carboxylic acid sometimes acts as a breakdown product from drugs like pyrazinamide, which plays a role in tuberculosis treatment. Knowing the formula and weight lets scientists track metabolites in blood samples, track down where a drug’s effects build up, and shed light on possible side effects. In my own work with heterocyclic compounds, I’ve seen how small changes in structure—like swapping a hydrogen for a carboxylic group—can shift activity by a mile. Predicting outcomes, measuring precisely, and following a molecule from synthesis to breakdown—formulas and weights make that possible.
Pyrazine-2-carboxylic acid does not exist just as an abstract compound; making and handling it raises questions of purity and safety. If you stumble on incomplete reactions or solvents that won’t leave, the formula and expected weight serve as a checkpoint. Analytical methods like mass spectrometry and nuclear magnetic resonance rely on knowing both the precise atomic arrangement and exact weight. Mismatched numbers or an unexpected peak can expose contamination before it risks the results.
Access to authenticated compounds, supported by transparent chemical registries and quality control, stays critical. Open collaboration between suppliers and researchers builds a chain of trust so that when you purchase a vial labeled “pyrazine-2-carboxylic acid,” its formula and weight match the certificate shipped with it. I’ve had my own experiments trip up when a compound turned out less pure than advertised, costing both time and credibility. Pushing for supplier transparency and detailed safety data helps labs everywhere. Routine validation keeps standards high and findings solid.
In the end, the simplest facts—like formula C5H4N2O2 and weight 124.10 g/mol—carry weight beyond tables and reference books. They make or break the way chemists think, prepare, analyze, and trust what happens in their flasks and eventually in healthcare. In my experience, attention to those details shapes progress and opens the door for discoveries people can count on.
When dealing with chemicals, keeping them stable over time isn’t just paperwork. It’s about protecting the people who use them and preserving their usefulness. Pyrazine-2-Carboxylic Acid, with its solid reputation in pharmaceutical research and analytical labs, fits the bill. Let the lid on the jar slip, and you’re no longer dealing with a reliable reference standard.
I’ve seen what humidity does to delicate compounds on a bad summer day: clumping, unpredictable results, sometimes a barely-there product left in the bottom of the bottle. Pyrazine-2-Carboxylic Acid holds up best in a cool, dry spot—ideally, 2–8°C in a tightly sealed container. This range matches most laboratory refrigerators. Warmer temps give the molecules more energy to react or degrade, and no one needs surprises during quality control or synthesis work. Excess moisture can lead to hydrolysis, slow but steady, which means the powder absorbs water and you can wind up with chemical changes you didn’t bargain for.
Direct sunlight isn’t just a problem for milk. Bright light can start breaking down sensitive aromatics and functional groups. This isn’t an abstract worry; I’ve pulled cloudy bottles from poorly shielded supply shelves and wondered how many experiments went wrong before anyone noticed. Keeping Pyrazine-2-Carboxylic Acid in amber bottles, or at least tucked away in a cabinet, stops light-induced reactions in their tracks. After that, minimize exposure to air by recapping tightly. Oxygen from the atmosphere slowly reacts with the compound’s ring structure over time, chipping away at both purity and performance.
Even with the best fridge and the darkest cabinet, an unlabeled bottle spells trouble. Always date the container and mark who opened it last. Segregation matters—don’t stack strong acids, alkalis, or oxidizers nearby. Each new chemical in a crowded storage space carries its own risks, especially if a shelf accident turns small spills into bigger problems. Being careful now beats cleaning up a mess later.
If stored cool and dry, Pyrazine-2-Carboxylic Acid generally remains stable for longer than a year. Still, I always recommend checking the certificate of analysis and looking for visible signs of change. Color shifts, moisture, or clumping serve as warnings. Don’t take chances on outdated stocks; recycling a few grams of chemical beats retracing months of failed data.
Many research groups run into cramped conditions and old equipment. Plenty of places fix this by investing in dedicated chemical refrigerators with temperature logs. Others use desiccators with silica gel packets to keep humidity impossible. It costs less than you might think to separate incompatible substances with simple plastic bins. Even on tight budgets, a clean, labeled, and organized shelf cuts down on risk, waste, and uncertainty.
Pyrazine-2-Carboxylic Acid stays relatively low on toxicological lists, but breathing in dust or accidental skin contact isn’t pleasant. Proper storage supports safe handling. Spills don’t only harm people—they can impact the environment or result in regulatory penalties. Combining safe storage with solid housekeeping means you aren’t just following the rules; you’re respecting your team and the planet.
Pyrazine-2-carboxylic acid does not pop up in most household conversations, but plenty of researchers and chemists recognize that name. The molecule shows up often during drug research, agricultural chemistry, and some industrial labs. Its structure is based on a pyrazine ring with a carboxyl group, making it pretty standard as far as aromatic acids go.
The best-known relationship between this chemical and the body appears in tuberculosis treatment. It comes from the breakdown of the drug pyrazinamide inside the body. When doctors prescribe pyrazinamide, the liver turns it into pyrazine-2-carboxylic acid, which then goes after tuberculosis bacteria. Researchers found this out through careful clinical studies. In my own experience working around infectious disease research, the way this breakdown shapes drug effectiveness matters a lot.
Pyrazine-2-carboxylic acid itself does not provide a direct threat to healthy cells at the doses found in medical treatment. Scientists checked blood levels in patients and tracked side effects; nothing scary came up in those results, so doctors kept using the parent drug.
Like most chemicals, too much of it can bring trouble. Test-tube studies suggest that at very high concentrations, there could be cell stress or irritation, but these levels do not show up during medication use or in typical laboratory settings. Animal testing—usually rodents—sometimes uncovers minor kidney tweaks at extreme dosages. Again, no one encounters these amounts outside a research lab with odd dosing ideas.
Human safety reports list basic precautions: standard gloves, goggles, careful handling. I remember my first time preparing solutions containing pyrazine derivatives; the instructions for safe handling sounded like any other organic acid—wash off spills, don’t eat or sniff, keep it labeled.
Government safety databases, such as the European Chemicals Agency or the U.S. National Institutes of Health, do not list pyrazine-2-carboxylic acid as a known carcinogen, mutagen, or major toxin. If this stuff spilled in a workplace, the cleanup would treat it the way any mild organic acid gets managed, no full hazmat suit required.
Pyrazine-2-carboxylic acid does not seem to stick around in the environment. Soil bacteria break it down, and it does not build up in water or living creatures. The modest solubility means a major spill dilutes quickly. Still, dumping high amounts in rivers or ground water would not win any environmental awards. Environmental laws make sure of that—no chemical, even “mild” ones, should go down the drain at scale.
In workplaces, good habits matter much more than scary warnings. Wearing gloves and washing hands goes a long way. Chemical suppliers write up a safety data sheet for every jar—simple steps make handling routine, not hazardous.
From a chemist’s point of view, the biggest thing about pyrazine-2-carboxylic acid is how it participates in drug action, not toxicity scares. Teachers, students, and industry workers should think of it as one more lab chemical—respect it, but don’t panic over it. Modern laws, carefully written instructions, and common sense provide all the tools needed to manage risk. Anyone handling this acid in a university, pharmaceutical, or agricultural lab can breathe easy, provided safety rules stick.
At a quick glance, a purity grade might seem like just a number. For anyone who has worked in a lab, that percentage tells a bigger story. Pyrazine-2-carboxylic acid, often trusted for research, drug development, and synthesis, typically shows up with purities from 95% up to 99%. There's even the rare 99.5% or higher, sometimes labeled as "ultra-pure" for the most demanding uses.
During my years working in research labs, chasing down the highest purity sometimes felt like chasing a unicorn. Suppliers offer "analytical grade," "reagent grade," and the rare "pharmaceutical grade." Each extra decimal point can eat into a grant budget. You pay for that kind of assurance because it can mean the difference between an experiment running smoothly or getting thrown right off by a stubborn contaminant.
A 95% bottle might look just as clean as one marked 99%. However, that extra four percent can hide surprising trouble. Impurities—those stray substances—act in unpredictable ways, especially in sensitive reactions. In pharmaceutical work, I’ve seen a trace impurity skew an assay, pushing a promising compound off the list just because it muddied the results.
If you're doing basic synthesis work, 95% usually gets the job done. For more sensitive tasks—pure drug preparation, HPLC analysis, sensitive catalytic reactions—you’ll want to step up to 98% or above. The food and drug sectors rarely gamble with anything below pharma-grade.
Manufacturers have to prove their product matches what they claim. High-purity pyrazine-2-carboxylic acid goes through rounds of spot checks, handled carefully to avoid introducing moisture and other contaminants during bottling. Labs like mine count on those certificates of analysis. There’s trust, but also verification.
Recent years have seen a gentle push from regulators for tighter standards—no one wants a recall because a batch turned out less pure than promised. China and India, both heavyweights in the chemical trade, set internal benchmarks and the most respected international suppliers aim to meet or exceed those.
It helps to know that higher purity doesn’t guarantee better results in every situation. Sometimes, a lower purity version works, with careful controls and good data backup. An experienced chemist spends as much time checking batch certificates as peering down a microscope.
Sourcing higher grades remains a challenge in regions cut off from major suppliers. Cost can block students or smaller labs. Open communication with wholesalers lets buyers explain what they need and why, opening the door to practical compromises, like blending a higher purity with a mainstream batch.
A drive for consistent quality raises the bar for everyone. Regulatory bodies, universities, and suppliers share a role. Better transparency and improved testing build a safety net, especially for young researchers learning the difference between "good enough" and game-changing.
Higher purity pyrazine-2-carboxylic acid isn’t just a badge for grants or regulatory gold stars. It's a practical choice that blocks bad data, missed opportunities, and even safety risks. Covet those certificates, ask tough questions, and—if doing groundbreaking work—never settle for less information than you deserve.
| Names | |
| Preferred IUPAC name | Pyrazine-2-carboxylic acid |
| Other names |
2-Pyrazinecarboxylic acid Pyrazinoic acid 2-Carboxypyrazine 2-pyrazinecarboxylate PCA |
| Pronunciation | /paɪˈræziːn tuː kɑːrˈbɒksɪlɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | [1123-00-8] |
| Beilstein Reference | 120843 |
| ChEBI | CHEBI:17911 |
| ChEMBL | CHEMBL1366 |
| ChemSpider | 18718 |
| DrugBank | DB04348 |
| ECHA InfoCard | 100.010.658 |
| EC Number | 207-934-3 |
| Gmelin Reference | 8595 |
| KEGG | C05914 |
| MeSH | D018047 |
| PubChem CID | 96824 |
| RTECS number | UY4375000 |
| UNII | 70J407ZL35 |
| UN number | Not regulated |
| Properties | |
| Chemical formula | C5H4N2O2 |
| Molar mass | 124.10 g/mol |
| Appearance | White to off-white powder |
| Odor | odorless |
| Density | 1.386 g/cm³ |
| Solubility in water | Slightly soluble in water |
| log P | -0.47 |
| Vapor pressure | 1.36E-7 mmHg at 25°C |
| Acidity (pKa) | 1.95 |
| Basicity (pKb) | 4.27 |
| Magnetic susceptibility (χ) | -33.4 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.620 |
| Dipole moment | 2.32 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 186.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -144.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1071.5 kJ/mol |
| Pharmacology | |
| ATC code | **J04AK07** |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: H315, H319, H335 |
| Precautionary statements | Precautionary statements: P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P312, P321, P330, P332+P313, P333+P313, P337+P313, P362+P364, P501 |
| Flash point | > 194.3 °C |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 4200 mg/kg |
| NIOSH | RN0025400 |
| PEL (Permissible) | PEL (Permissible exposure limit) for Pyrazine-2-Carboxylic Acid is not established. |
| IDLH (Immediate danger) | IDLH not established |
| Related compounds | |
| Related compounds |
Pyrazine 2-Aminopyrazine 2,3-Pyrazinedicarboxylic acid 2-Hydroxypyrazine Isonicotinic acid Nicotinic acid |