Chemists started paying attention to pyrazine-based compounds in the late 1800s, driven by curiosity about aromatic heterocycles and their reactivity. Early experiments scattered across Europe and North America mapped the landscape of pyrazine derivatives, with pyrazine-2,3-dicarboxylic acid standing out for its two carboxyl groups sitting on a rigid, nitrogen-rich ring. During the mid-20th century, synthetic organic chemistry picked up pace, making this compound regularly accessible in university labs and industrial pilot programs. Years of research pushed boundaries, not only defining structure and reactivity but also linking chemical features to practical uses in pharmaceuticals, materials science, and analytical chemistry. My own brush with its history came during a graduate seminar, where a professor recounted struggles separating dicarboxylic isomers before chromatography matured, illustrating the tough journey from obscure synthesis to regular laboratory use.
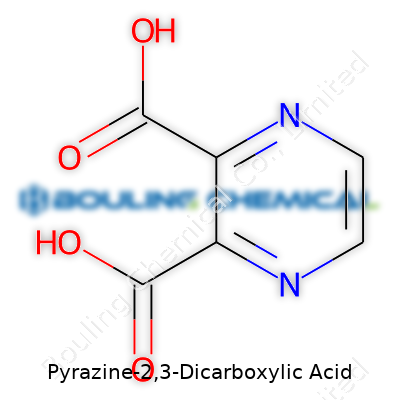
Pyrazine-2,3-dicarboxylic acid draws attention for its straightforward, logical atomic arrangement. The pyrazine ring—a six-membered aromatic skeleton with nitrogen at positions 1 and 4—attaches carboxylic groups at positions 2 and 3, carving out a molecular identity distinct from other ring systems. Suppliers list the molecule as a white to off-white crystalline solid, shipping by the gram or kilogram depending on the order. Lab researchers often buy it as a starting material for building more complex molecules or in screening studies aimed at identifying lead candidates for new medicines. Long shelf life, modest storage demands, and well-documented reactivity contribute to its appeal among those who want reliable intermediates for synthesis work.
Anybody who’s handled pyrazine-2,3-dicarboxylic acid quickly notices its robust crystalline texture and relatively high melting point, typically hovering between 320°C and 325°C. The odorless properties stem from the strong aromatic framework and the absence of volatile side groups, ensuring a stable bench presence even if storage space gets cramped. Its solubility in water stays low, while exposure to dimethyl sulfoxide or basic aqueous solutions reveals a marked uptick as deprotonation comes into play. The acidity of both carboxylic groups hovers around moderate pKa values, which means one can push and pull hydrogens off with reasonably mild acids or bases. Chemically, the aromatic ring remains resistant, except in the hands of a skilled chemist using potent oxidants or reducing agents. This stability creates a platform for methodical modification rather than explosive, unpredictable reactions.
Manufacturers publish technical sheets listing key identifiers: molecular formula C6H4N2O4, molecular weight 168.11 g/mol, and relevant analytical standards like IR, NMR, and Mass Spectrometry fingerprints. Standard labels include the CAS registry number 89-01-0, purity levels reaching 98% or higher, and instructions for ambient temperature storage away from strong acids or bases. Transportation falls under Class 6 for toxic substances if shipped in bulk, although routine lab purchases skirt this mark. Reliable labeling helps avoid confusion, especially in shared academic or industry spaces where dozens of white powders might fill one shelf. From experience, sourcing from trusted suppliers cuts down on headaches like contamination or mismarked vials, both of which can sidetrack a project before it even hits the hood.
Classic procedures for making pyrazine-2,3-dicarboxylic acid often start from commercial diaminomaleonitrile or pyrazine-2,3-diamine, introducing carboxyl groups through oxidation and hydrolysis steps under carefully controlled conditions. In one recipe I read, a mixture of potassium permanganate, water, and precursor simmers under reflux, yielding a crude acid that can be recrystallized from ethanol or water. Some newer syntheses rely on catalytic coupling methods or milder oxidants, prioritizing fewer waste byproducts and greener chemistry. While it may take a few hours from start to purified product, the chemistry rarely surprises those with basic organic training. Cleanup, though, can demand persistence, as residual color and unwanted inorganic sludges tend to stick around unless filtered out with care.
Pyrazine-2,3-dicarboxylic acid lends itself to a toolbox full of reactions. Esterification, amidation, and dehydration all proceed predictably. Those carboxyls can react with alcohols or amines under classic coupling conditions, letting you swap out acidic hydrogens for tailored side chains. The aromatic core resists many attacks, yet under strong enough conditions, one can coax out chlorination or bromination with wet halogen reagents, though yields rarely impress unless you baby every step. Reductive decarboxylation splits off CO2 to make simpler pyrazines, while selective reduction yields dihydropyrazine analogs. In my own attempts to spin out library compounds for medicinal screening, this acid proved far more responsive than expected under palladium-catalyzed coupling, suggesting room for further creative modification with modern cross-coupling methods.
Ask a vendor or read a chemical database, and you’ll run into plenty of aliases: Dipicolinic acid, Pyrazine-2,3-dioic acid, and 2,3-Pyrazinedicarboxylic acid show up most often, though I’ve seen quirky in-house names like “PDCA” crop up in some research papers. Cross-referencing these synonyms matters when checking literature or ordering from suppliers that operate in different regions. Clear nomenclature reduces blunders—nobody wants to find out mid-experiment they got a methylated derivative. Safety Data Sheets almost always double-list common names and registry numbers, an obvious nod to the compound’s multiple identities across commercial, academic, and regulatory settings.
Handling pyrazine-2,3-dicarboxylic acid requires baseline laboratory safety, though its general profile reads less severe than notorious hazards like cyanides or peroxides. Inhalation, skin, or eye contact deserves avoidance—personal protective equipment covers these risks well. Glove use and eye shielding form basic protocol for weighing and solution prep. Spilling small amounts warrants cleanup with damp towels or appropriate waste bins; for larger quantities, local regulations push for special chemical spill kits and ventilation. Every responsible workplace keeps digital and print Material Safety Data Sheets on hand, a habit drilled into me during my years in teaching labs. Waste disposal channels this acid into regulated containers, never down the drain, protecting both pipes and people. Regular audits from environmental health and safety officers keep everyone honest in settings where even minor spills matter to compliance.
Lab chemists value pyrazine-2,3-dicarboxylic acid as a scaffold for new drug candidates, agricultural agents, and materials suited to advanced ceramics or polymer reinforcement. Pharmaceutical groups test derivatives as anti-infectives or anti-inflammatory compounds, exploring the ring’s electronic effects in binding studies. In materials science, coordination chemistry taps into the molecule’s two carboxyls, letting it bridge between metals and build intricate frameworks with plenty of industrial promise. Researchers in environmental science test this acid in metal-chelation protocols, especially in trace analysis settings where cleaner complexation streams lead to crisper results. My own work involved binding this acid to rare earths, stabilizing luminescent complexes for use in analytical kits. Each time I circled back to literature, new application ideas bubbled up, signaling broad and growing interest beyond a single discipline.
Current R&D programs dig deeper into both classic reactivity and emerging technologies. Teams map the landscape of pyrazine-2,3-dicarboxylic derivatives, modifying positions on the ring to nudge activity, solubility, and selectivity. Drug discovery groups screen libraries built from this acid using high-throughput robotic systems, feeding hits into computational models predicting binding to protein pockets or enzyme active sites. Green chemistry researchers aim to fine-tune preparation protocols and recycle waste streams, nudged by both economics and tightening regulations. Multi-institutional projects spark collaboration between academic labs in heterocyclic synthesis and pharmaceutical discovery groups, proving that innovation rarely respects institutional boundaries. Some of the most promising developments come from coupling this acid to nanomaterials, a strategem shown to shape particle surface chemistry and behavior in water.
While not typically classified as a major toxin, pyrazine-2,3-dicarboxylic acid draws scrutiny from toxicologists seeking to map safety limits and chronic exposure effects. Animal studies measure LD50 values well above acute risk thresholds, though certain metabolic breakdown products warrant careful monitoring. Human data remain scarce, mainly because lab exposure stays low and ingestion risks tend to zero under normal handling. Environmental scientists watch for downstream effects if disposal enters water supplies, prompting targeted tests for bioaccumulation and aquatic toxicity. Regulatory filings in the US and EU underline the need for ongoing monitoring—not out of alarm, but out of the precautionary principle. Seasoned researchers keep exposure minimal, using fume hoods or local exhaust in large-scale preps, carrying forward a culture of careful chemical stewardship.
Looking ahead, pyrazine-2,3-dicarboxylic acid sits at a promising intersection between reliable chemistry and bold discovery. Synthetic chemists eye it both as a springboard for complex heterocycles and as a resilient linker for building supramolecular assemblies or flexible polymers. As analytical tools become more sensitive, its subtle reaction products and metal complexes could fuel advances in imaging, diagnostics, and environmental sensing. Demand for sustainable syntheses and safer derivatives stirs chemists to tweak existing routes, reducing hazardous reagents and improving atom efficiency. The drumbeat for cross-disciplinary breakthroughs doesn’t quiet—biologists, engineers, and material scientists add new twists to this compound’s evolving story each year. For those of us who value a steady partner for scientific improvisation, pyrazine-2,3-dicarboxylic acid promises a long runway of invention, bounded only by the imagination and persistence of those willing to run the next experiment.
My first encounter with Pyrazine-2,3-dicarboxylic acid happened in a university lab, where some students were busy discussing the relevance of heterocyclic compounds. It stood out as more than just another obscure molecule in a catalogue. I learned that chemists often prize its structure for its twin carboxyl groups flanking a nitrogen-heavy pyrazine ring. This makes it an essential starting point for building more complex chemicals, especially for pharmaceutical research.
It's fascinating how small molecules like this one can trigger a wave of new discoveries. Researchers draw on Pyrazine-2,3-dicarboxylic acid to construct various compounds, particularly when searching for new antibiotics or anti-inflammatory drugs. This isn’t hype; literature backs up these claims. For example, studies published in “Journal of Medicinal Chemistry” show that pyrazine derivatives contribute vital core structures for drug candidates targeting infections and chronic diseases.
In transition metal chemistry, this compound acts like a reliable team player. Scientists often use it to form coordination complexes with metals like copper or zinc. These complexes handle electron transfer smoothly, so they fit perfectly in applications such as catalysts for redox processes or as materials in sensors. Coordination chemistry can look abstract from the outside, but anyone who's worked on analytical devices or water purification systems knows how these complexes push real progress on the ground.
Beyond health and chemistry, Pyrazine-2,3-dicarboxylic acid steps into the world of advanced materials. Researchers have used it as a linker for creating metal-organic frameworks, or MOFs, which store gases or filter chemicals from air and water. Scientists published in “Chem. Soc. Rev.” describe how tweaking linkers like this one can change how efficiently a MOF traps carbon dioxide or separates toxic industrial fumes. Given the global push for sustainable technology, materials that improve air and water quality attract growing attention, both in research circles and in industry.
Industry barely gets by without versatile molecules like Pyrazine-2,3-dicarboxylic acid. Bulk chemical manufacturers reach for such intermediates to build pesticides and dyes. Many of these applications fly under the public’s radar but impact the food supply and consumer products. For those working in chemical safety or environmental monitoring, some of the synthetic routes and byproducts pose challenges. Open conversations about process safety and waste disposal remain critical as production scales up.
Real progress with molecules like this one requires collaboration among chemists, toxicologists, and environmental experts. Producers can work on greener synthesis methods, reducing hazardous reagents and minimizing waste. Open-access data on toxicity and environmental persistence will help regulatory agencies make informed decisions. Chemists in academia and industry can share best practices to increase safety and reduce costs, creating a ripple effect that benefits the whole supply chain.
It’s clear that Pyrazine-2,3-dicarboxylic acid isn’t a household name, but its fingerprints are all over key advances in medicine, materials science, and industrial chemistry. Recognizing its value means appreciating both the breakthroughs it enables and the responsibility that comes with using it wisely.
Pyrazine-2,3-dicarboxylic acid stands out in a lab for its straightforward yet impactful structure. It’s built from a pyrazine ring, which is a six-membered aromatic ring with two nitrogen atoms sitting across from each other, and two carboxylic acid groups hooked onto the second and third carbon atoms. The molecular formula for this compound is C6H4N2O4. Measuring weight in chemistry means weighing every atom, and this one lands at a molecular weight of 180.11 g/mol.
Chemists look at molecules the way craftsmen look at their tools. The two carboxylic acid groups in Pyrazine-2,3-dicarboxylic acid open up possibilities. With carboxylic acids, you can form salts, esters, or amides. In my time working on heterocyclic chemistry, that versatility meant I could tweak reactivity or lock in specific behaviors for catalysts or dyes. In the pharmaceutical world, scaffolds like this one often serve as backbones for more complex drugs, sometimes showing antibacterial or enzyme-inhibiting action. Papers in journals like Journal of Medicinal Chemistry have chronicled how pyrazine derivatives anchor new drug research
Analyzing molecules starts with counting atoms. For Pyrazine-2,3-dicarboxylic acid:
Every chemist remembers the sting of wrong weights on a scale. In industrial labs, using the wrong molecular weight means faulty batches and lost money. In pharmaceutical research, these numbers affect dosage, toxicity predictions, and even patent filings. According to the FDA’s chemical database, accuracy in basic chemical data sits at the base of everything from early screening to later product approvals.
This compound rarely gets the media spotlight, but it’s not just a bench-top oddity. Researchers use it in metal-organic frameworks, which help scientists trap gases or design smart sensors. I’ve met peers who struggled with scale-up because suppliers gave off-spec material—either wrong formula or moisture-laden samples. Reliable sourcing platforms like Sigma-Aldrich stress the value of checking every detail through certificates of analysis. Labs thrive on trust, and open data-sharing makes that possible.
Fixing accuracy issues goes beyond the lone chemist. Standardized database entries, cross-checked by both humans and algorithms, limit mistakes. Open-access databases like PubChem or ChemSpider make it easier to verify molecular formulae and weights. Encouraging companies to share purity reports or batch analysis data builds confidence. Direct training in quality-checking also saves research time and money. Mistakes in chemistry hurt, but with the right checks, anyone can avoid them—and discover something new along the way.
Chemicals like Pyrazine-2,3-Dicarboxylic Acid do not forgive carelessness. Many research labs keep their shelves full of various compounds, hoping each stays as pure as the day it arrived. Storing this acid the wrong way leads to ruined experiments, safety risks, and wasted money.
This compound, known to some as dipicolinic acid, plays a major role in science—especially for folks working in pharmaceuticals, materials, and analytical chemistry. Stability keeps its value intact. Moisture, heat, and light can gradually wear it down, changing both look and performance. These aren’t just theoretical problems. I’ve seen entire shipments turn useless after someone put chemical jars next to a window. Anyone responsible for rare or costly reagents learns fast: storage isn’t an afterthought.
Lab workers and anyone storing fine chemicals should aim for a dry, cool, and dark spot. Pyrazine-2,3-Dicarboxylic Acid draws in water from the air, which means humidity quickly turns it into a sticky mess or changes weight readings. A desiccator, kept well-stocked with fresh desiccant, protects it. I once made the mistake of tossing a poorly capped bottle into a regular cupboard. The next time I opened it, clumps and discoloration greeted me.
Direct sunshine does more damage than most folks realize. Light can spark unwanted reactions inside the jar. Even fluorescent bulbs pump out plenty of energy to boost degradation. Stash this chemical in amber bottles or a closed opaque cabinet to slow these invisible threats.
A constant, moderate temperature works best. Extreme heat pushes breakdown reactions, while a freezer introduces unwanted water when the container gets repeatedly opened and closed. A simple shelf away from heat vents, radiators, or sunbeams keeps most research-grade acids stable, and Pyrazine-2,3-Dicarboxylic Acid is no exception.
Sealing the jar tightly always helps, especially if you swap out air for an inert gas like nitrogen. For most labs, though, keeping the cap snug and oxygen out delivers good results. Glass works best, especially with a screw cap that has a liner. Plastic sometimes leaches chemicals, so glass brings more peace of mind.
Label the bottle with the date received and the opening date. Marking these avoids mistakes. I remember a time when two nearly identical jars caused confusion, thanks to sloppy handwriting. It saves a lot of double-checking if you keep everything clear from the start.
Safety rules are written in red ink for a reason. Always store acids away from strong bases and organics, even if space feels tight. Pyrazine-2,3-Dicarboxylic Acid reacts with some materials if spilled or mixed accidentally. Wearing gloves and goggles during transfers isn’t just about compliance; it keeps skin and eyes unscathed.
If in doubt, consult a detailed chemical safety data sheet from a reputable supplier. Resources from Sigma-Aldrich, Thermo Fisher Scientific, and public health agencies set the gold standard. Training new staff on these principles saves money and nerves in the long run.
Every bottle of Pyrazine-2,3-Dicarboxylic Acid becomes an investment. Taking care with humidity, temperature, and contamination holds value for months or years. Thoughtful storage habits protect research, budgets, and above all, people—lessons learned from scars and success stories alike.
Pyrazine-2,3-dicarboxylic acid finds its way into a handful of lab benches thanks to its chemical flexibility. Researchers sometimes study it for its unique structure or as an ingredient in making metal-organic frameworks. This is not some flavoring you find in food or an additive in cleaning products. Most people never encounter it outside of a chemistry lab or specialty industrial setting. Still, curiosity about its safety keeps popping up, especially when lab teams stumble across it in older syntheses or specialty catalogs.
No one wants to take chances around new substances. The facts on pyrazine-2,3-dicarboxylic acid give a pretty clear picture. Peer-reviewed research and chemical safety databases, including the European Chemicals Agency (ECHA) and PubChem, list it as a solid with low volatility. It doesn’t put out toxic fumes under normal room conditions. Compared to many lab chemicals, that’s a plus.
But that doesn't mean care stops. This compound, like almost all organic acids, causes eye and skin irritation. Breathing in dust will probably leave a sore throat or cough. If you mix it with strong oxidizers or acids, you might get an unwanted reaction. I remember a colleague splashing a similar organic acid on his hand. He shrugged it off at first, then spent the rest of the afternoon nursing red, puffy skin. Old school chemists didn’t always bother with gloves—these days, no one risks it.
Looking at toxicity, no studies suggest pyrazine-2,3-dicarboxylic acid causes cancer, birth defects, or chronic organ harm. Most databases mark it as having low acute toxicity by ingestion or skin contact, though no one volunteers to test that. Some older animal studies place it in the category of “irritant, not especially dangerous,” if handled with routine lab protocols. The acid doesn’t dissolve readily in water, so spills won’t sweep through groundwater or rivers. Nonetheless, lab manuals stress treating all chemicals with respect—wear goggles, use gloves, and work in a fume hood.
Even with the rulebook, some areas stay blurry. Few long-term studies exist. Regulatory bodies don’t list this molecule under special restrictions, mainly because it’s not in wide commercial use. That lack of deep research means that assessment focuses on general chemical sense—use it as intended, with normal controls. It’s not a green light to drop standards. One careless pour down the sink or a dusty bottle left wide open creates problems not only for the individual but for custodial staff cleaning up the workspace.
Anyone working with pyrazine-2,3-dicarboxylic acid should read through the safety data sheet, keep it labeled, and store it away from food and strong oxidizers. Personal protective equipment—the basic gloves, goggles, and lab coat—does the heavy lifting. Clean up with plenty of running water for spills. Store waste for proper disposal rather than tossing it with regular trash. Teaching safety in school labs sticks with people long after graduation. These habits matter for any lab, any project, any chemical—even those like this one, sitting on the lower end of the hazard spectrum. Keeping things safe in the workplace boils down to preparation and respect for the unknown.
Pyrazine-2,3-dicarboxylic acid sounds like a mouthful, but its significance can’t be ignored in research labs and certain chemical industries. This organic acid, found as a white to off-white powder, gets a place in synthesis projects and as a building block for more specialized molecules. What stands out most, beyond its tongue-twisting name, is the purity.
In my experience, purity makes or breaks a reagent’s usefulness. Most scientists or production engineers eye a minimum of 98% purity for Pyrazine-2,3-dicarboxylic acid. Labs spend a fair bit checking certificates of analysis and asking suppliers about quality control. Any drop below that, and unwanted side products start sneaking into results.
HPLC, NMR, and melting point tests tell the real story of what buyers get. Sometimes, manufacturers push the purity up to 99%—a nice number for anyone who wants to cut down on troubleshooting. If you’ve ever wasted hours tracking down mysterious interference in an experiment, you know that one percent can make a mountain of difference.
Smaller labs and big plants use materials at different rates. Pack sizes for pyrazine-2,3-dicarboxylic acid follow this real-world demand. You won’t catch a research bench buying anything in drum quantities—most academic work sticks to 1 gram, 5 grams, or maybe 25 grams.
Scale-up work, pilot production, or routine industrial processes call for larger volumes. I’ve seen standard packaging ranging from 100 grams to 1 kg or even up to 25 kg fiber drums. Heavy bags or drums come with tamper-proof seals and double-layer lining because nobody needs moisture or dust in their chemicals.
Some suppliers customize packaging, and this flexibility really helps prevent material waste. No one likes paying premium for extra reagent that just expires on the shelf. Still, most requests settle on those standard sizes—big enough for productivity, not so much that storage turns into a headache.
Storage and shipment give another layer of challenge. Even with high purity, poor packaging can lead to clumping or slow degradation. I always check for clear labeling—lot number, manufacture date, and safety hazards right on every bag or bottle. A missing label can turn a routine day into a scavenger hunt.
Some companies, especially those selling internationally, use moisture-proof, light-resistant containers. Over years of ordering fine chemicals, I’ve learned to appreciate double-bagging and vacuum seals more than any shiny catalogue. For fine organic acids, air and sunlight are the enemy. Keeping a lid on these risks saves both money and time in repeated orders.
Transparency in purity and packaging goes a long way to reduce mistakes. Industry bodies and quality standards like ISO keep everyone honest, but buyers still need to double-check every order. Building a relationship with reliable suppliers—ideally those willing to share detailed analysis—cuts down on rejections and gives peace of mind.
No fancy slogan or glossy brochure replaces a clean batch with proper paperwork. For labs and plants using pyrazine-2,3-dicarboxylic acid, information on purity and pack size is more than housekeeping—it's core to safe, accurate, and efficient operations.
| Names | |
| Preferred IUPAC name | Pyrazine-2,3-dicarboxylic acid |
| Other names |
2,3-Pyrazinedicarboxylic acid 2,3-Dicarboxypyrazine Pyrazine-2,3-dioic acid |
| Pronunciation | /paɪˈræziːn tuː θriː daɪˈkɑːksɪlɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 89-01-0 |
| Beilstein Reference | 128969 |
| ChEBI | CHEBI:37980 |
| ChEMBL | CHEMBL2106021 |
| ChemSpider | 167045 |
| DrugBank | DB04260 |
| ECHA InfoCard | ECHA InfoCard: 100.005.272 |
| EC Number | EC Number: 219-422-5 |
| Gmelin Reference | 79040 |
| KEGG | C16275 |
| MeSH | D003437 |
| PubChem CID | 98997 |
| RTECS number | UY9100000 |
| UNII | F4VQU76N44 |
| UN number | Not regulated |
| CompTox Dashboard (EPA) | IVWQHEOXJNXLJK-UHFFFAOYSA-N |
| Properties | |
| Chemical formula | C6H4N2O4 |
| Molar mass | 180.12 g/mol |
| Appearance | White to light yellow crystalline powder |
| Odor | Odorless |
| Density | 1.667 g/cm3 |
| Solubility in water | Slightly soluble in water |
| log P | -1.5 |
| Vapor pressure | 1.28E-7 mmHg at 25°C |
| Acidity (pKa) | 1.08 |
| Basicity (pKb) | pKb = 0.82 |
| Magnetic susceptibility (χ) | Magnetic susceptibility (χ) of Pyrazine-2,3-Dicarboxylic Acid: -54.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.674 |
| Viscosity | 1.44E1 mPa·s (20°C) |
| Dipole moment | 2.37 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 186.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -618.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1114.3 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | Precautionary statements: P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P337+P313 |
| NFPA 704 (fire diamond) | NFPA 704: 1-1-0 |
| Flash point | > 316.5°C |
| Lethal dose or concentration | LD50 oral rat 3450 mg/kg |
| LD50 (median dose) | LD50 (median dose) = 5000 mg/kg (Rat, Oral) |
| NIOSH | SE1961600 |
| PEL (Permissible) | PEL (Permissible exposure limit) information for Pyrazine-2,3-Dicarboxylic Acid is not established. |
| REL (Recommended) | 10 mg/m3 |
| Related compounds | |
| Related compounds |
2,3-Pyrazinedicarboxylic acid monohydrate 2,6-Pyrazinedicarboxylic acid Pyrazine Pyridine-2,3-dicarboxylic acid Quinoxaline-2,3-dicarboxylic acid 2,3-Pyridinedicarboxylic acid |