Chemists have long searched for molecules that bring unique effects in both industry and research labs. Piperidinium chloride shows up early in classical chemistry writings, dating back to the era when bicyclic amines drew attention for their stability and reactivity. It found its early place through basic amination reactions, especially once synthesis routes for piperidine became routine. Labs in Europe and North America quickly caught onto its potential. By the mid-20th century, improvements in purification and scaling-up meant that research groups could routinely analyze its behavior in simple salt forms. This story is about more than just a molecule. It traces shifts in industrial chemistry—how researchers learned that a simple quaternary ammonium compound could touch everything from solvents to drug-making.
Piperidinium chloride comes across as a reliable quaternary ammonium salt built from a piperidine backbone and chloride anion. In practical terms, it shows up in bottles and bags as a powdered or crystalline solid, usually white in color. Companies sell different grades depending on purity required—lab grade, industrial grade, or pharmaceutical-permitted quality. Each batch comes labeled with molecular structure, lot number, and purity details so scientists and plant operators stay on top of the specifics during their experiments or manufacturing.
This salt looks unassuming, but its physical traits reveal serious chemistry. It carries a molecular formula of C5H12NCl and a molecular weight just above 121 grams per mole. The crystals dissolve well in water, show limited solubility in organic solvents, and will melt if temperatures push beyond 250°C. The compound starts to break down only at much higher temperatures or under conditions that challenge the nitrogen–carbon bonds. It holds up against light, doesn’t absorb water easily, and stands stable in sealed containers out of direct sunlight. Researchers like the predictability, since reliable handling reduces errors and allows careful control of stoichiometry in syntheses.
Suppliers frame their technical sheets with clear numbers: typical assays run greater than 98% purity, chloride content meets international standards, and residual moisture stays below 0.5% for best performance. Labels describe hazard codes and reactiveness, based on criteria published by OSHA and ECHA. A standard container lists chemical name, CAS number, lot code, manufacturer ID, storage guidance, and date of packaging. These details aren’t filler—they help track shipments, recall products when problems arise, and prove compliance for audit teams watching over pharmaceutical or agricultural operations.
Synthesizing piperidinium chloride starts with piperidine, often made by reduction of pyridine or cyclization of hexamethylenediamine. Chemists react piperidine with hydrochloric acid gas or concentrated HCl solution, which leads to precipitation of the chloride salt. The mixture gets cooled, filtered, and often recrystallized for extra purity. Labs will analyze the final product through titration, infrared spectroscopy, and sometimes elemental analysis to guarantee a quality meeting end-user requirements. For plant-scale work, twin-screw reactors and batch tanks handle most of the workflow, with waste streams directed to acid-neutralization steps. Safety teams demand closed systems wherever possible, since HCl gas and free amines carry hazards for lungs and skin.
Piperidinium chloride doesn’t just sit idle; it opens doors in both synthetic and catalytic chemistry. For basic research, it serves as a phase-transfer catalyst, moving reactants between aqueous and organic layers. Its nitrogen center allows for N-alkylation, Hofmann elimination, and even the formation of ylides used in organic syntheses. Medicinal chemists use the piperidinium cation as a scaffold for drugs—quaternization offers a way to tweak solubility or bioavailability in candidate drugs. Modifications can involve swapping the counterion, introducing alkyl or aryl groups onto the ring, or even replacing the nitrogen’s hydrogen with complex organic chains. Each change unlocks versatility, especially for pharmaceutical, agricultural, and analytical products.
You’ll see piperidinium chloride referred to in supplier catalogs and chemical inventories under several names—N-piperidinium chloride, hexahydroazepinium chloride, and 1-piperidine hydrochloride stand out as leading synonyms. CAS numbers help sort out confusion: regulatory bodies recognize CAS 626-20-0 as the official tag. Some vendors market custom blends or stabilize the product with silica to prevent caking, but at core, the chemical identity remains the same.
Working with piperidinium chloride requires attention to safety straight from the container’s first opening. Although acute toxicity isn’t high at typical handling concentrations, mishandling can irritate skin, eyes, and lungs. Splash goggles, nitrile gloves, and fume hoods make up the frontline. Larger operations lock in containment systems, real-time air quality monitors, and written protocols addressing mix-ups or spills. Transport falls under ADR and DOT regulations for hazardous chemicals, backed by documentation and proper labeling. First aid includes flushing with water, while emergency response teams know to control spread using absorbents, then segregate wastes for licensed disposal. Plant managers don’t aim for risk-free; they design procedures reducing exposure and respond fast if something goes off-script.
Lab folks, industrial chemists, and pharmaceutical crews each approach piperidinium chloride with their own goals. In organic synthesis, it serves as a building block for functionalized amines, peptidomimetics, and heterocycles. The compound plays a role in making phase-transfer catalysts, chiral auxiliaries, and antiarrhythmic drug candidates. Agricultural teams use it for plant growth regulators, insecticides, and herbicide intermediates. Analytical chemists exploit its predictable ion-pairing for trace detection or instrument calibration. Industrial sites find it valuable for specialty coatings and treating resins, where small changes in amine structure alter performance across coatings, inks, and adhesives.
Current research pushes the envelope for both synthetic routes and targeted applications. Some teams develop greener synthesis methods—looking at solvent-free protocols, recyclable catalysts, and even enzymatic transformations for environmental gains. Structural analogues of piperidinium chloride find use as templates in drug discovery, with teams from Asia to Europe reporting advances in antiviral and anticancer compounds. Materials science groups experiment with the salt in polymer blends for battery membranes and composites. Partnerships between universities and industry help move promising results from flask to field, with documentation available through patents, technical bulletins, and the open literature.
Scientists still dig into the short- and long-term effects of piperidinium chloride exposure. Animal studies suggest mild acute toxicity, mostly at high doses and through direct injection or inhalation. Chronic exposure hasn’t shown up as a big danger in the workplace, provided operators follow standard controls. In soil and water, breakdown comes slowly unless environmental bacteria step up, which leads researchers to call for waste containment and careful downstream monitoring. Regulatory groups such as the EPA and ECHA assign piperidinium chloride moderate hazard ratings, with emphasis on limiting environmental release and tracking workplace air concentrations. Toxicologists push for better data, especially as new derivatives enter pharmaceutical pipelines.
The future looks busy for piperidinium chloride and its chemical cousins. With new focus on sustainable synthesis, companies pursue lower-waste methods and renewable starting materials. Product design stretches into fine chemicals, energy storage, and drug delivery platforms. Academic labs continue exploring the structure–activity links that make the piperidinium ring valuable in medicinal chemistry. Regulatory frameworks continue to tighten, calling for greater transparency on sourcing, purity, and safe use. For practitioners and innovators alike, it’s not just about keeping up with advances; it’s about setting new standards in performance, safety, and responsibility.
Piperidinium chloride starts off sounding a lot like something that belongs in a secretive lab or the plot of a TV drama. The reality tells a more useful story—this compound does real work in industrial and research settings. Its roots trace back to piperidine, a building block found in natural alkaloids and known for its distinct, fishy smell. Tweak piperidine just a bit and you get piperidinium, a salt with a reputation for reliability in chemistry circles.
Chemists count on stable substances for processes like organic synthesis. Piperidinium chloride stands out because it brings both stability and reactivity, which helps reactions move in the right direction. It acts as a catalyst in some situations, often as a phase-transfer agent. Its main job is to help molecules mingle when they’d rather stay separated, speeding up reactions that otherwise crawl along at a snail’s pace. Cleaner outcomes and less waste matter for both health and the environment.
Extraction and purification work rely on compounds like piperidinium chloride. In drug development and fine chemical manufacturing, you only want the good stuff—no side products or leftovers. This chemical has proven itself by giving cleaner cuts and saving both time and energy. These aren’t theoretical benefits; drug companies and manufacturers have documented more consistent yields and fewer fails when piperidinium chloride gets involved.
Nobody likes surprises in a pill bottle. The pharmaceutical industry demands steadiness and accuracy. Piperidinium chloride sometimes helps produce active ingredients or serves as a helper compound during synthesis. Regulatory agencies, from the FDA to the EMA, demand a lot more than a simple list of ingredients—they inspect every detail, making sure nothing toxic or unpredictable gets through. Years in research labs have shown that piperidinium chloride’s role, while unglamorous, helps make that supply chain safer.
Drug safety stays top of mind. Research from safety watchdog groups and published journals back up the substance’s safety record when used under controlled circumstances and handled by professionals. Those safety numbers offer some peace of mind to manufacturing workers and scientists alike.
The value of piperidinium chloride comes with a need for care. It’s not something for casual use in an unventilated room. Overexposure may trigger respiratory irritation, so companies invest in gloves, goggles, and proper ventilation for a good reason. I’ve worked in labs where checklists and training weren’t just bureaucratic rituals—they kept us alert and healthy. Science doesn’t move forward by ignoring caution.
Training and transparency set a clear path forward. Factories and labs that store or move chemicals update their material safety data sheets regularly and stress a “see something, say something” culture. These habits don’t just check boxes: they keep people out of the doctor’s office and keep shipments moving safely.
Greener processes show real promise for replacing some harsh chemicals with gentler ones, or at least reducing quantity and exposure. Some chemists aim to swap in piperidinium chloride for older, dirtier alternatives. Success stories come out of universities that share their data openly—step by step, the industry adapts, choosing routes that protect both workers and the planet. Watching chemicals like piperidinium chloride make that journey isn’t just a technical improvement; it reflects a broader pattern of listening to both science and people’s experience in the workplace.
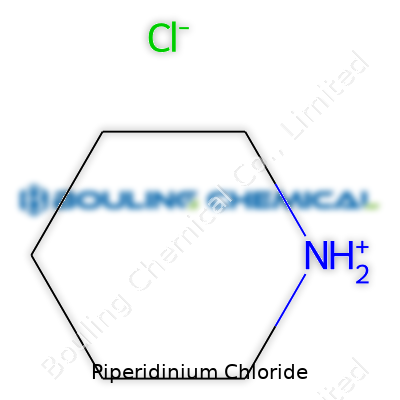
Piperidinium chloride carries the chemical formula C5H12NCl. In this salt, the piperidinium ion links up with a chloride ion. Piperidinium itself comes from piperidine, a six-membered nitrogous ring that shows up in many everyday products, medicines, and lab experiments. Adding a hydrogen ion to piperidine produces the piperidinium cation, then pairing with chloride makes piperidinium chloride, a white crystalline substance that dissolves easily in water.
A formula on paper only tells part of the story. I've personally seen plenty of confusion among students and professionals trying to distinguish between the piperidine base (C5H11N) and its chloride salt. Getting the formula wrong means risking costly mistakes in the chemistry lab, where even one atom can change the behavior of a reaction. My time teaching undergraduate chemistry taught me how formulas like C5H12NCl aren't just trivia — they're the instructions behind which chemicals you actually have and how they act.
This molecule pops up in synthetic chemistry. Researchers use it as a starting point to build more complex structures, many with pharmaceutical importance. Misunderstanding the exact composition leads to failed syntheses and wasted resources. Last year, a former colleague worked on a drug candidate that needed a positively charged nitrogen. Choosing piperidinium chloride kept reactions simple and avoided surprises with side reactions, since the chloride acts as a straightforward counterion.
Accurate chemical identification underpins lab safety. Mixing up piperidine and piperidinium chloride brings more than a textbook error — one is a volatile amine, the other a more stable salt. Serious labs check every shipment and label, double-checking formulas before anything touches a beaker. The stakes are high: mistakes like the wrong formula can mean exposure to hazardous vapors or reactions going dangerously awry.
Beyond the bench, paperwork depends on the right formula. Regulatory bodies want exact identification for every material. Products that hit the market must clear hurdles from agencies such as the FDA or EPA. If someone's filing lists the wrong compound, it delays research and can trigger costly recalls.
Learning starts with writing down the right formula. I encourage students to practice translating names to formulas and vice versa. Piperidinium chloride, with its straightforward makeup, offers a good challenge: you look for the C5 five-carbon ring, the saturated hydrogens, the nitrogen, and the single chloride. It trains attention to detail — a skill that flows through every part of scientific work.
Industry can help by clear labeling and training on interpreting chemical names. Software that cross-checks names and formulas before chemicals are ordered or used reduces the risk. People who work with these compounds every day benefit from reference charts, tutorials, and examples that stick close to the molecules in question.
With the right chemical formula for piperidinium chloride in hand, chemists, students, and industry have a better shot at safe, effective, and innovative work in the lab and beyond.
I’ve worked around labs where chemicals like Piperidinium Chloride show up in drawers and storage cabinets. Folks sometimes think that if a compound isn’t covered in bold hazard labels, it doesn’t present a threat. That’s not true—especially in tightly controlled lab environments. Piperidinium Chloride can irritate skin, lungs, and eyes. Breathe in its dust, and your airways complain. Splash it on your hands without gloves and you get irritation that usually sticks around for hours. Eyes feel the sting right away. Don’t assume this chemical plays nice just because it isn’t an acid or an explosive.
Not enough people respect the role of good gloves, face shields, and goggles. Nitrile gloves handle most exposures from Piperidinium Chloride, so I’ve made it a habit to inspect them first for tears or signs of wear. If you’re transferring powder or preparing solutions, dust can rise in the air fast. Toss on a lab coat and chemical goggles. For folks with facial hair, goggles fit better than safety glasses, which leave skin exposed. Respirators don’t always feel comfortable, but a properly fitted N95 or P100 stops those tiny particulates from finding your nose or mouth. It pays to keep a mask handy even for brief jobs.
I’ve cleaned up spills before where someone left a scoop sitting loose on the bench, bits of Piperidinium Chloride powder scattered across the surface. Accidents like this creep up fast, and too many people get complacent. It takes only a few seconds to seal the container tight and wipe down the workspace. Handwashing comes next—soap, warm water, scrubbing the backs of your hands and under the nails. Never eat or drink in the workspace. Once you touch chemicals, they cling. A meal break at the lab bench puts you at risk. Take off lab coats before grabbing lunch.
I once watched a beginner weigh out Piperidinium Chloride outside a fume hood, thinking the task was too quick to matter. Strong airflow pulls dust and vapors away before they can reach your breathing zone. Always set up your work inside a hood or beneath an extractor. Storing Piperidinium Chloride requires dry, sealed containers—moisture degrades quality and raises risks of clumping or spills. Keep storage spaces cool and well-ventilated. I’ve learned that investing in well-marked, dedicated cabinets pays off by keeping incompatible chemicals separate.
Don’t wait for an accident before you check where the nearest eyewash or emergency shower stands. Practice quick paths to exits and know the spill kit contents. For minor skin contact, running water calms irritation if you start rinsing immediately. If powder hits the eyes, flush for at least fifteen minutes. Report all spills so the next person isn’t caught off guard. Contact local poison control or occupational health experts for bigger incidents. Quick responses save headaches—and sometimes something worse.
Building a safer culture starts with sharing experiences and clear communication. Lab managers need to encourage questions—no shaming for double-checking a procedure or pausing to put on safety gear. Hands-on training and real-life examples stick much stronger than endless safety posters. Piperidinium Chloride won’t cause problems if you treat it with the same respect you’d give any hazardous compound. In my experience, the little habits like wiping down a workspace or checking gloves before use hold just as much weight as the formal training sessions. Safety grows from the bottom up, lived out one careful preparation at a time.
From years spent working in chemical labs—big universities, small startups, even at home—I've seen what can happen when people get too relaxed about storing chemicals. Piperidinium chloride might not be a household name, but it shows up often in research and in certain manufacturing processes. If labs treat handling and storage like an afterthought, things can go sideways in a hurry. Protection, health, and maintaining sample quality come down to more than just rules on paper.
Direct sunlight and moisture change a substance in ways folks underestimate. Even with the best intentions, busy labs leave containers on benches or under bright lights. Piperidinium chloride breaks down more quickly when heat or humidity gets involved. Decomposition means impure samples, unexpected reactions, or even dangerous fumes over time. I once opened a container left near a sink—the smell gave away the mistake instantly. It only took a few hours of forgotten care to lose a vital batch.
Cool, consistent temps prevent problems. Chemical storage areas rely on roof vents, fans, or even climate control to keep the air moving and the temperature steady. Piperidinium chloride fares best in a space around 20-25°C, away from both freezing drafts and heaters. Big labs use refrigerator units for sensitive samples, but not every operation can afford that kind of setup. Even a steel or plastic cabinet, placed far from hot air vents or windows, gives some peace of mind.
A solid screw-top bottle stops vapors escaping or dust sneaking in. Over the years, I’ve watched people use whatever empty jar sits closest. Chemically resistant materials—typically polyethylene or glass—make a difference. Lids need intact liners and clear labeling. I’ve come across mystery substances in reused jars more than once; nobody wants the stress of guessing at contents if labels fall off. A printed label with the chemical’s name, plus hazard warnings, keeps things legal and safer for everyone handling it.
A lot of accidents start with the wrong person reaching for the wrong flask. Locking cabinets don’t just comply with lab safety audits—they actually prevent mistakes. Undergraduate labs and shared spaces have more foot traffic, more hands reaching into storage. The simple step of locking hazardous materials blocks curious visitors, untrained helpers, and even the odd absent-minded scientist.
Even the best plan meets surprise. I review spill kits every few weeks, checking gloves, absorbent pads, and emergency shower readiness. Piperidinium chloride isn’t flammable, but spills can irritate eyes and skin. One small leak, unattended, coats a shelf and lingers for months if missed. Immediate cleanup and waste disposal stop a bad day from getting worse. Regularly reviewing safety data sheets helps everyone up to speed, especially newcomers or rotating lab workers.
Storing Piperidinium chloride isn’t about avoiding a citation or ticking off a checklist. It’s about building habits that protect everyone in the building. Proper storage lowers the odds of spoiled products, lost money, and unsafe exposures. A well-kept inventory, cool storage, sealed bottles, and good signage create an environment where science happens safely—not just fast. Sharing this mindset across teams, from senior researchers to newcomers, protects projects and people equally.
Piperidinium chloride has a utility most folks never hear about. It shows up in specialty chemical synthesis, certain types of research, and occasionally in industrial processes. People working with it see more than just a bag of white powder—they see a tool for labs, something with chemical bite. Like most powerful tools, it asks for respect.
Chemicals in the piperidinium family aren’t strangers to debate. According to research published in peer-reviewed journals and safety data sheets (SDS), this compound brings risks if it finds its way into your lungs or touches skin for any length of time. Scientists at OSHA and the European Chemicals Agency have flagged its irritating properties. Short exposure usually leads to redness or a burning feeling. Breathing it in can stir up coughing and discomfort. I once watched a lab colleague get a mild skin rash after careless handling—not exactly a medical emergency, but enough to ruin a day’s research.
It’s not just surface-level irritation, either. Chronic exposure hasn’t been studied much in large populations, but animal studies tip us off: higher doses over time can turn into nervous system impacts and problems with organs like the liver. That uncertainty makes me think twice about proper gloves, lab coats, and ventilation, especially since exposure rules are not always well-enforced.
Disposing of piperidinium chloride gives environmental scientists a tough problem. If it flows into rivers or soil, the story doesn’t end at the test tube. Early earthworm toxicity screens suggest it breaks down slowly. That means extra pressure on plants and bugs. Aquatic life seems especially vulnerable. Fish studies highlighted disrupted swimming patterns and stunted early development. I grew up fishing local streams, so contamination feels personal. Runoff from poorly managed labs or factories suddenly stops being invisible.
Chemicals fill water and dirt fast if protocols break down. Small spills stick around longer than most hope. So, every academic lab and factory tracking hazardous materials must keep tight records and stick to disposal plans that involve trained waste handlers, not regular trash. Clean water and healthy land matter not just on spreadsheets, but in people’s daily lives. Most regulations in the US and Europe say hazardous chemicals, including related nitrogen compounds, belong in expert hands—never poured down drains or tossed in bins.
Hazards linked to piperidinium chloride aren’t some distant, theoretical topic. In my early research days, seeing a chemical burn taught me to respect labels and keep the eyewash station uncluttered. Labs that drill staff in safety see fewer injuries. Encouraging a direct attitude—no shortcuts, no missed training days—brings positive change.
Moving forward, industry and universities can do better. Investing in robust monitoring, keeping chemical storage up-to-date, and supporting staff training helps keep problems in check. Some countries reward safe practices with reduced insurance premiums or public recognition. Industry groups have started to publish “safer substitution” guides, urging the shift to less hazardous materials when research allows. That’s tough at first but worth attempting, even if it means rethinking protocols or spending more on new supplies.
Piperidinium chloride brings value to modern chemistry, but it’s not innocent. Safety precautions, respect for ecosystems, and open conversations with regulators make for a safer future.
| Names | |
| Preferred IUPAC name | piperidin-1-ium chloride |
| Other names |
1-Piperidinium chloride Piperidine hydrochloride Piperidinium chloride (1:1) Piperidinium monohydrochloride |
| Pronunciation | /paɪˌpɛrɪˈdɪniəm ˈklɔːraɪd/ |
| Identifiers | |
| CAS Number | 660-68-4 |
| 3D model (JSmol) | `P([Cl-])(C1CCNCC1)` |
| Beilstein Reference | 1361688 |
| ChEBI | CHEBI:64046 |
| ChEMBL | CHEMBL1230979 |
| ChemSpider | 13472 |
| DrugBank | DB08483 |
| ECHA InfoCard | 100.146.618 |
| EC Number | 205-793-5 |
| Gmelin Reference | 7641 |
| KEGG | C07343 |
| MeSH | D010901 |
| PubChem CID | 23868453 |
| RTECS number | TJ6475000 |
| UNII | K49WLP65G6 |
| UN number | UN2810 |
| CompTox Dashboard (EPA) | DTXSID2023137 |
| Properties | |
| Chemical formula | C5H12ClN |
| Molar mass | 120.62 g/mol |
| Appearance | White to almost white crystalline powder |
| Odor | fish-like |
| Density | 1.053 g/cm3 |
| Solubility in water | Very soluble in water |
| log P | -3.6 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 11.2 |
| Basicity (pKb) | 2.80 |
| Magnetic susceptibility (χ) | -63.8 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.4700 |
| Viscosity | Viscous liquid |
| Dipole moment | 3.49 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 143.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -263.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | Std enthalpy of combustion (ΔcH⦵298) of Piperidinium Chloride: -3656 kJ/mol |
| Pharmacology | |
| ATC code | N04AA02 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Autoignition temperature | 355°C |
| Lethal dose or concentration | LD50 oral rat 570 mg/kg |
| LD50 (median dose) | 1000 mg/kg (rat, oral) |
| NIOSH | SKC350 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 250 mg/m3 |
| Related compounds | |
| Related compounds |
Piperidine Piperidinium bromide Pyridinium chloride Tetraethylammonium chloride Tetramethylammonium chloride |