People in chemistry circles have paid close attention to piperidine-N-carbaldehyde for decades, tracing its origins back to classic heterocyclic research. Early studies in organic chemistry often focused on finding new ways to build ring structures like piperidine, a backbone in countless alkaloids and synthetic drugs. With advances in reagents and purification, chemists extended their curiosity to piperidine-N-carbaldehyde, especially as aldehyde chemistry flourished in the mid-20th century. Labs in academic corridors piloted early syntheses, reporting yields and side products in journals that shaped experimental best practices. As industrial demand for intermediates surged in fields like agrochemicals and pharmaceuticals, companies scaled up production, and safety documentation improved, thanks in part to lessons from earlier laboratory mishaps and the gradual tightening of industry oversight.
Piperidine-N-carbaldehyde stands as a colorless to pale yellow liquid at room temperature. Its distinctive odor gives away its aldehyde nature, a warning sign for experienced chemists who respect its reactive formyl group. As a precursor, it gives plenty of options in synthesis, forming stable bonds with nitrogen and allowing versatile transformations. The compound rarely features in household products, yet specialists recognize its value in research and industrial labs for constructing a range of heterocyclic frameworks used in medicines, crop protection, and advanced materials. Popular suppliers package it in glass or high density polyethylene, checking purity through established assays so chemists can safely predict outcomes in sensitive multistep reactions.
At a glance, piperidine-N-carbaldehyde’s structure features a six-membered ring capped by a reactive aldehyde group on nitrogen, which causes the molecule to stray from planarity and introduces unique electronic behavior. It usually boils in the range typical for similar heterocyclic aldehydes, hovering just below 90°C under reduced pressure. Solubility stays high in most common organic solvents; water only dissolves modest amounts, limiting its use in purely aqueous systems. Its volatility, paired with strong reactivity of the aldehyde, keeps storage under anhydrous, cold conditions essential. Shelf stability depends on protection from air and moisture—over time, it oxidizes or polymerizes, especially if left exposed.
Project outcomes in manufacturing or research rely on reliable material quality, so suppliers always note key numbers on packaging: CAS registry number, molecular weight (113.16 g/mol), chemical formula (C6H11NO), and expected purity, which frequently hits 98% or higher. Safety warnings include flammability, volatility, and a strong recommendation for protective gear, since accidental exposure carries health risks. Standard labeling, guided by the Globally Harmonized System, includes pictograms showing health hazard and chemical exclamation. Lots often include lot number, manufacture date, and expiration recommendations, which helps in quality audits and research reproducibility.
Most production routes rely on starting with piperidine and introducing a formyl group directly to the nitrogen. Common methods include the Vilsmeier-Haack reaction, which starts with formyl chloride or dimethylformamide and a Lewis acid to achieve the transformation under controlled temperatures. Post-reaction, mixtures need neutralization, extraction, and drying, often under nitrogen. Crude product usually undergoes vacuum distillation to tease out pure piperidine-N-carbaldehyde and leave behind less-volatile impurities. Waste streams, especially containing chlorinated intermediates, demand careful neutralization and disposal, underscoring responsibilities around chemical stewardship in modern practice.
Due to the presence of both a nucleophilic amine and an electrophilic aldehyde, this compound supports a broad canvas of reactions. Reductive amination turns it into secondary amines, often using sodium cyanoborohydride or catalytic hydrogenation, a mainstay in pharmaceutical labs making active substances. Aldol condensations and other addition reactions open pathways to fused heterocycles—these backbone structures show up in many therapeutic agents. Derivatization by acylation or alkylation fine-tunes electronic properties for use as ligands in homogeneous catalysis, where selectivity and reactivity mean everything for an industry that thrives on yield optimization and waste minimization.
A search through commercial catalogs and regulatory lists uncovers multiple names: 1-Piperidinecarboxaldehyde, N-Formylpiperidine, Piperidinylcarbaldehyde, and even catalog simplifications such as Piperidinecarbaldehyde. Within regulatory paperwork, the correct structure always matters, as confusion can trigger safety missteps or legal issues during procurement and auditing. This multiplicity of names reflects both chemistry’s long habit of evolving nomenclature and the pressures of international trade and compliance.
Labs familiar with the hazards of low-molecular aldehydes stick to fume hoods and tight protocols. Skin and eye contact risk strong irritation, and inhalation of vapors may trigger headaches, coughing, or more severe respiratory effects. Spills get cleaned quickly with absorbent, non-reactive materials. Storage avoids sunlight, heat, and sources of ignition, and emergency procedures mandate showers and eyewash stations nearby. Waste collection points use labeled, chemical-resistant containers, and regular disposal pickups—no room for improvisation when working with chemicals flagged as hazardous, especially where regulatory inspections or workplace safety reviews could spring up at any time.
In pharmaceutical research, the role of piperidine-N-carbaldehyde reaches beyond mere transformation. Chemists use it to introduce unique motifs for drug candidates targeting neurological, infectious, and metabolic disorders. Crop science outfits look for analogs that stunt pest development by disrupting neurotransmission. Watchful overproduct quality, analysts use the compound as a derivatizing agent in chemical analysis, sometimes for quantifying amino groups or tracking elusive metabolites. Specialty chemical producers explore its assembly into dyes, stabilizers, and even advanced polymers. Each application leans on the distinctive reactivity, occasional volatility, and the ease with which this molecule can slip into complex synthetic sequences.
Each year, published studies reference piperidine-N-carbaldehyde in new ways. Academics describe conditions that push up the yield, or cut down on byproducts, while industry patents detail reaction sequences that shave hours off process time. Teams at chemical companies track competitor breakthroughs, eager for clues that could boost selectivity or allow greener, less wasteful routes. Analytical chemists refine detection methods—gas chromatography, mass spectrometry—so even trace impurities reveal themselves early enough to correct courses. Through collaboration, improvements in catalysts, solvents, and even reactor technology create possibilities for using this compound under more sustainable, energy-efficient conditions, extending its reach across high-value sectors.
Research dives deep into the toxicity profile, because handling any low-boiling aldehyde brings inherent risk. Acute inhalation studies flag narcotic effects and respiratory system irritation at higher concentrations, based on rodent and cell culture data. Data from in vitro genotoxicity screens keeps regulators cautious but indicates no direct mutagenic risk when managed with basic precautions. Long-term exposure guidelines now inform workplace limits in much of the world, so exposure gets tracked with badge samplers and air monitoring. Training drills keep staff engaged, not complacent, knowing the price of a careless week in the lab can mean lasting health impacts or costly fines.
With green chemistry pulling more weight in the industry, the search intensifies for synthesis routes that cut out toxic byproducts and lower the carbon footprint. Automated continuous flow reactors might soon replace batch processes, slashing waste and boosting yields for lab and plant alike. As drug discovery and advanced materials research demand more customized heterocycles, flexible building blocks like piperidine-N-carbaldehyde grow more valuable. Renewed interest in sustainable agriculture and pest management introduces pathways to next-generation plant protectants. Researchers will likely keep tuning purity, packaging, and application parameters to meet tougher standards in environmental and personal health protection, making innovation in safe handling as important as reaction yield.
Walk into a chemistry lab, and you spot shelves full of liquids and powders, all labeled with names that sound more like tongue twisters. Now, Piperidine-N-Carbaldehyde is one of those names you won’t find on a supermarket shelf or in your medicine cupboard, but it plays a noticeable role behind the scenes in chemical synthesis.
Chemists use Piperidine-N-Carbaldehyde as a building block. It features a piperidine ring, which pops up in several bioactive compounds and mediation research. The big deal about it is its placement in forming custom molecules for drug design and discovery. Drug developers lean on such intermediates to alter and refine new medicines. Building blocks like this allow scientists to piece together new molecules, test their properties, and tweak their effects until they find promising results that may address diseases or conditions that we care about.
Over the years, advances in synthetic chemistry opened doors to target compounds with higher accuracy. Research papers highlight Piperidine-N-Carbaldehyde’s use as a step toward creating certain antihistamines, antidepressants, and even drugs aimed at neurological disorders. For example, piperidine rings show up in muscle relaxants and blood pressure medications. Piperidine-N-Carbaldehyde’s reactive form makes it valuable in piecing together these larger, more complex drugs. Anyone working in a medicinal chemistry lab has likely handled aldehyde intermediates as part of a broader effort to improve health outcomes.
On the other side, questions might pop up about why interest in such chemicals isn’t just limited to the science world. Chemicals with features like a piperidine ring sometimes draw attention from those looking to make illicit substances. Synthetic routes for certain banned drugs can use related intermediates along the way. This has led regulatory agencies to monitor products like Piperidine-N-Carbaldehyde, especially in countries facing problems with synthetic drug production. Laboratories have to track inventories and follow strict protocols to avoid misuse.
It’s a tough spot for honest researchers. Restrictions sometimes mean legitimate science slows down. In my graduate days, delays while waiting for certain permitted chemicals could derail months of planning in the lab. At the same time, no one wants unchecked availability fueling criminal activity.
Balancing progress and safety isn’t just a slogan in chemistry; it’s a lived experience for people who work in the lab. Some organizations now use tighter tracking systems, improving accountability. National drug agencies have responded by developing clearer guidelines to ensure that chemicals like Piperidine-N-Carbaldehyde are used by vetted professionals. Real solutions depend on keeping legitimate users in the loop and discouraging those with other intentions.
Better communication is starting to help. Education programs in universities stress not just what chemicals do, but why ethical usage matters. Early-career scientists learn about record keeping, safety protocols, and legal responsibilities. This builds trust between regulators and researchers, making it easier for everyone to work toward breakthroughs without opening new risks.
Piperidine-N-Carbaldehyde might look like a strange, purely technical concern at first. Behind the name, though, are layers of complex science, human choices, regulatory challenges, and the constant pressure to keep research both safe and productive.
Piperidine-N-carbaldehyde rarely shows up in general conversations, but folks who work in chemistry labs recognize the name. This compound comes with a set of risks that might seem like a distant concern until a spill happens or a small mistake leads to a big problem. Every year, industry reports and academic journals turn out plenty of stories about chemical incidents due to overlooked hazards. This one fits right in with compounds needing serious respect.
I’ve worked around chemicals long enough to know that a complex name doesn’t always signal serious danger, but in this case, there are reasons to be concerned. Like many organic aldehydes, Piperidine-N-carbaldehyde can irritate the skin, eyes, and respiratory tract. Getting a little on your hands isn’t just uncomfortable — it can cause redness, swelling, and itching. Breathing in even small amounts may trigger coughing or difficulty breathing. The evidence isn’t just anecdotal; safety data sheets from reputable chemical suppliers detail these effects.
Sometimes, it’s not the splash or the sniff that causes trouble. Consistent, low-level exposure tends to creep up on people who spend long hours in the lab. Chronic exposure isn’t well studied for this chemical, but looking at similar compounds, organ damage and sensitization aren’t out of the question. That news hits differently if you’ve ever worked beside someone who didn’t take glove protocols seriously until it was too late.
Best practices for dealing with Piperidine-N-carbaldehyde line up with those for other hazardous chemicals. Fume hoods aren’t just suggestions — they are a line of defense. Nitrile gloves, proper eye protection, and emergency eyewash stations have saved people from painful, expensive mistakes. Cleanup plans matter, not because of rare explosions or Hollywood-style disasters, but because most chemical injuries happen from small spills and inattentive moments.
Uncontrolled disposal causes more headaches than immediate spills. Once this compound washes down the drain without proper treatment, it moves into waterways, where it poses toxicity risks to aquatic life. Regulatory guides from organizations like the EPA and OSHA make it clear that such chemicals belong in carefully handled waste streams. Simple shortcuts can lead to expensive fines and community pushback.
Laboratories, both academic and industrial, keep relying on new and specialized compounds for innovation. The problem isn’t only the molecule itself — it’s how fast people push forward without enough training on new risks. Stories from the field show that even veteran chemists make mistakes when they underestimate a chemical’s potential danger. Real safety depends on habits: reading the SDS before opening a bottle, labeling containers clearly, and never leaving a reaction unmonitored.
Toxicity isn’t limited to acute symptoms or rare events. It’s wrapped up in casual mistakes and moments of neglect. Sharing real incidents, tracking near-misses, and checking in with coworkers about procedures builds the kind of culture that prevents injuries. Training and support need to keep pace with new compounds like Piperidine-N-carbaldehyde. Vigilance costs less than one trip to the emergency room or the loss of trust in a team that didn’t take safety seriously enough.
Piperidine-N-Carbaldehyde pops up in the world of organic chemistry as a useful building block, prized for its role in forming bonds and shaping molecules. Its chemical formula, C6H11NO, says a lot. Six carbons, eleven hydrogens, one nitrogen, and a single oxygen come together here, forming a molecule with almost endless possibilities for chemical reactions.
Here, the backbone of the structure is a piperidine ring—think of a six-membered ring, mostly saturated, with a single nitrogen atom taking the place of one carbon. At the nitrogen position, there’s a carbaldehyde group. That means the nitrogen of the ring is linked to a CHO group (the classic mark of an aldehyde). This combination pushes Piperidine-N-Carbaldehyde into an interesting spot both in research labs and industrial plants.
Structure tells the story. The nitrogen atom in the piperidine ring carries a lot of chemical weight. In the presence of the aldehyde group, nucleophilic attacks become accessible, allowing the compound to take part in a range of coupling reactions and syntheses. It’s not just theory—this behavior shows up in labs every day. My own experience with similar ring structures backs this up. Functionalized piperidine derivatives often serve as intermediates for new pharmaceuticals because they lay a solid foundation for tweaking drug activity and metabolism.
Piperidine derivatives aren’t a niche interest. More than 60 medications listed by the FDA contain this ring, each taking advantage of how the basic ring supports chemical modification. The aldehyde at the nitrogen in Piperidine-N-Carbaldehyde makes it an even more versatile precursor for further transformations—Schiff base formation and reductive amination open doors for organic chemists.
Actual synthesis of this compound usually involves formylation of piperidine with specific reagents, often deploying Vilsmeier–Haack or similar aldehyde-forming chemistry. Yields and purity depend on controlling reaction conditions, which speaks to the hands-on side of chemical synthesis—a process any organic chemist knows well.
Connecting piperidine rings to aldehyde groups streamlines the creation of diverse compounds. Medicinal chemists look to Piperidine-N-Carbaldehyde not because it’s rare or exclusive, but because its chemistry fits smoothly into existing reaction schemes. The molecule can help introduce functionalities into larger frameworks, supporting research on central nervous system agents and anti-infectives.
Safety and handling also cannot be ignored. Aldehyde groups can cause irritation and require careful storage in well-ventilated areas, often under inert gas. Companies and labs must adopt strong protocols to protect researchers, prevent contamination, and stay compliant with chemical safety regulations. Exposure to the vapors can irritate eyes and mucous membranes, so investing in the right personal protective equipment protects more than just projects—it protects people.
Looking for solutions, chemists should keep working on greener synthesis routes and safer handling techniques. Advances in continuous flow reactors, for example, stand to offer efficiency, reduce waste, and improve scalability. Academic and industrial partnerships can push innovation faster, sharing protocols and data.
As the demand for piperidine-based drugs and advanced materials grows, Piperidine-N-Carbaldehyde sticks around as a cornerstone, reminding chemists of the power held by small tweaks to a molecule’s shape. Every lab experiment or synthetic trial brings the global scientific community a step closer to safer, more effective compounds.
My own work in academic chemistry labs taught me quickly that chemicals like Piperidine-N-Carbaldehyde demand attention. This isn’t the sort of material you toss on the nearest shelf or forget in a drawer. The compound's reactivity and volatility put it on the hazardous list at nearly every research institute. Safety starts from how you store it.
A big lesson from real lab breakdowns: storing Piperidine-N-Carbaldehyde in a cool, dry area saves both property and peace of mind. Leave a bottle too close to hot equipment or a sunny window, and you’re risking degraded material, nasty vapors, or worse. Standard operating procedure tells us to keep these materials well below room temperature, often in specially marked chemical refrigerators. Not every fridge qualifies. Only those designed to handle flammable or reactive chemicals really fit the bill.
Don’t count on a loosely sealed bottle. I once opened a cabinet and caught a whiff of aldehydes because someone thought a bit of parafilm was “good enough.” Proper storage involves tightly sealed glass containers with screw caps. Anything less lets moisture and oxygen creep inside, speeding up decomposition and sending unwanted fumes around the room. In some places, high-quality polyethylene bottles also make the list, as long as the manufacturer says they’re up for the job.
Any lab director with a few years under the belt will tell you — never stick hazardous chemicals in a neglected corner. A ventilated storage cabinet, ideally one with chemical-resistance and exhaust connections, makes a difference. Storing Piperidine-N-Carbaldehyde away from incompatible materials (especially oxidizers and acids) reduces accident risk. Separate shelves or bins, clear signage, and chemical inventory logs reinforce those boundaries.
The list of controlled chemicals in some countries includes Piperidine-N-Carbaldehyde because of its potential use in illegal synthesis. Keeping it in a locked cabinet isn’t just a regulation — it stops theft or accidental misuse. Only authorized personnel should access or handle it. On more than one occasion, careful tracking of every milliliter headed off bigger problems and helped meet strict audit requirements.
The best storage plans prepare for the worst. Spills happen, as I learned cleaning up after a toppled shelf. Absorbent pads, chemical spill kits, gloves, and masks need to stay close, not buried under paperwork. Piperidine-N-Carbaldehyde has a pungent odor and irritating vapor, so workers have to stay alert for off smells or bottle discoloration — both signs it’s time to dispose of stock using proper hazardous waste protocols.
New staff and students only understand the risks if training goes beyond a quick walk-through. Sharing real incidents — like the time a cracked cap ruined a freezer batch — makes proper storage more memorable. Labels with clear hazard warnings aren’t optional, and regular reviews keep everyone on the same page.
Solid habits, good equipment, and honest, ongoing safety training usually beat expensive technology for preventing accidents or loss. Keeping Piperidine-N-Carbaldehyde under close watch, with the right conditions and controls, lets everyone get the work done and head home safe.
I’ve worked with plenty of reagents over the years, and piperidine-N-carbaldehyde lands firmly in my memory as a chemical that asks for real respect. Sure, it’s not the flashiest name, but it’s not exactly harmless either. Getting overconfident—or sloppy—with it asks for trouble. The smell alone signals you’ve got something more than plain water, but the problems run deeper than your nose. It can irritate your eyes and skin, cause respiratory issues if you breathe in fumes, and create toxic effects if you’re careless. Some researchers blow off proper gear, maybe thinking a single exposure won’t hurt. That’s a fast track to a bad day.
No one wants to wrestle with goggles and gloves all afternoon, but for piperidine-N-carbaldehyde, skipping PPE isn’t clever. Nitrile gloves actually save you—some folks reach for latex, but that’s not reliable. Full-seal goggles keep splashes and fumes out of your eyes. Lab coats and long sleeves make a difference too, especially when splatters sneak up on you. Years ago, I watched a colleague brush off the need for a face shield and end up with a burned patch on his forearm. It took weeks to heal. You skip layers only if you’re planning to learn the hard way.
Good ventilation pays for itself, and you want that chemical moving away from your breathing space. Fume hoods aren’t decorative—they defend your lungs. If you’ve ever caught a whiff outside a hood, the harsh, biting scent leaves your throat raw. Shutting the sash and keeping work inside the airflow cuts the risk, plain and simple. In smaller operations or cramped university labs, skipping the hood can lead to headaches, dizziness, or worse. It’s not just theory. A colleague who bid farewell to his undergraduate days told me about a time he handled small volumes at a bench and spent the afternoon coughing. Management learned the lesson after the fact, not before.
Keeping the bottle tightly sealed, clearly labeled, and stored away from oxidizers or acids beats dealing with a spill or a runaway reaction. I’ve seen forgotten bottles in college refrigerators that cracked over time—cleaning that slop proves far nastier than handling it freshly stocked. Separate flammables from everything else; don’t trust chance with something reactive. That’s not just my experience—years of chemical incident reports back that up. Regulators like OSHA have published lists of cases where poor storage led to real harm—damage to property and health.
Training stands out as the most straightforward fix. Supervisors think a handout covers it, but walking through procedures and showing safety setups beats reading about it. Spills need more than a quick mop; absorbents and proper disposal cut the spread of contamination. Clear labeling, constant reminders, and having an emergency shower nearby stack the deck in your favor if things go south. The more you act on these habits, the less excitement you’ll have—and in labs, boring is best.
People sometimes treat safety as a hindrance. From what I’ve watched, the extra five minutes to suit up or double-check a label can keep you off the accident roster for the year. That’s something every lab should remember.
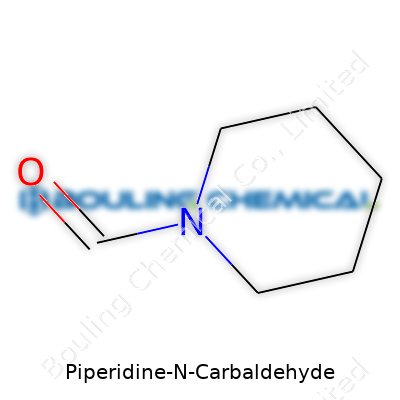
| Names | |
| Preferred IUPAC name | piperidine-1-carbaldehyde |
| Other names |
1-Piperidinecarboxaldehyde Piperidine-1-carbaldehyde Piperidin-1-carbaldehyde Piperidine aldehyde |
| Pronunciation | /paɪˈpɛrɪdiːn ɛn kɑːrˈbældɪhaɪd/ |
| Identifiers | |
| CAS Number | 22255-07-6 |
| 3D model (JSmol) | `C1CCCNC1C=O` |
| Beilstein Reference | 1209241 |
| ChEBI | CHEBI:139444 |
| ChEMBL | CHEMBL497444 |
| ChemSpider | 57732 |
| DrugBank | DB08278 |
| ECHA InfoCard | 03b7f4bf-887c-45bc-b578-9c7f587fe81d |
| EC Number | 1.5.3.1 |
| Gmelin Reference | 85324 |
| KEGG | C06252 |
| MeSH | D010876 |
| PubChem CID | 12502 |
| RTECS number | SS8400000 |
| UNII | MBR8V3F19K |
| UN number | UN3276 |
| CompTox Dashboard (EPA) | DTXSID60892265 |
| Properties | |
| Chemical formula | C6H11NO |
| Molar mass | 99.13 g/mol |
| Appearance | Colorless to yellow liquid |
| Odor | fishy |
| Density | 0.911 g/cm³ |
| Solubility in water | Soluble |
| log P | 0.35 |
| Vapor pressure | 0.6 mmHg (at 25°C) |
| Acidity (pKa) | 11.12 |
| Basicity (pKb) | 2.88 |
| Magnetic susceptibility (χ) | -10.05 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.457 |
| Viscosity | 0.893 cP (20°C) |
| Dipole moment | 2.6051 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 309.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -24.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3466 kJ/mol |
| Pharmacology | |
| ATC code | N05CM19 |
| Hazards | |
| GHS labelling | GHS02,GHS05,GHS07 |
| Pictograms | C1CCCNC1C=O |
| Signal word | Warning |
| Hazard statements | H226, H302, H312, H314, H332 |
| Precautionary statements | Precautionary statements: P210, P261, P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 3-3-2-W |
| Flash point | 67 °C (153 °F; 340 K) |
| Autoignition temperature | AUTOIGNITION: 385°C |
| Explosive limits | Lower explosive limit: 1.4%, Upper explosive limit: 8.4% |
| Lethal dose or concentration | LD50 oral rat 1640 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 500 mg/kg |
| NIOSH | NIOSH: RV3675000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Piperidine-N-Carbaldehyde is not specifically established by OSHA. |
| REL (Recommended) | 1 mg/m3 |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Piperidine Pyridine Piperidinecarboxaldehyde Piperidine-4-carboxaldehyde Piperidine-N-oxide 2-Piperidone N-Methylpiperidine Piperidine-2-carboxylic acid |