Chemists started exploring derivatives of piperidine as soon as they realized the core piperidine ring could serve as a bridge for many pharmaceutical and chemical innovations. In the 20th century, once structural analysis tools and synthetic organic chemistry matured, folks gave special attention to modified piperidines like 3,5-dimethylpiperidine. By the 1970s, labs across Europe and North America recognized this compound’s possible value for drug discovery and agrochemicals. Publications from the mid-century pop up with trial syntheses and reactivity notes, marking its slow but steady entrance into wider chemical catalogs.
Sitting on the shelf in a standard amber bottle, 3,5-dimethylpiperidine looks like a small player in chemistry, but it forms a real backbone for advanced molecule design. This molecular workhorse contains a six-member ring, bearing two methyl groups at positions 3 and 5, making it a handy building block for researchers shaping new pharmaceuticals or specialty materials. You run into it most by catalog number in lab supply lists, where it comes in both racemic and enantiopure versions, depending on the downstream applications.
3,5-Dimethylpiperidine, as a nitrogen-containing heterocycle, sits as a colorless to pale yellow liquid or sometimes as a solid, depending on the storage temperature. At standard conditions, its boiling point lands close to 150–160°C, showing decent volatility but not so much that it evaporates overnight. The compound’s odor—sharp and amine-like—makes its presence known fast on an open bench. It dissolves well in organic solvents like ethanol, ether, and chloroform, yet it shies away from water, echoing many of its alkylated cousins with similar ring structures. Stability remains high under neutral conditions, but strong acids or bases tear into the ring, breaking it down with vigor.
In any compound warehouse, 3,5-dimethylpiperidine usually carries a label with CAS number 35794-11-7 and a molecular formula C7H15N. Many research-grade samples offer a purity greater than 98%, tested by gas chromatography and nuclear magnetic resonance spectroscopy. Labels flash signal words like "Warning" or "Caution," along with pictograms for skin and eye irritants. A breakdown of batch number, synthesis date, and shelf life accompanies documentation, direct from suppliers focused on traceability and user safety. Transport falls under the basic rules for organic amines, avoiding pressurized containers and direct sunlight to keep leaks and decomposition at bay.
Laboratories commonly prepare 3,5-dimethylpiperidine through hydrogenation of 3,5-dimethylpyridine. This process, run under mild pressures with a palladium or platinum catalyst, offers high yields and selective reduction without too many headaches. The starting pyridine comes from methylation of standard pyridine rings—a routine transformation in academic and industrial labs. Once hydrogenation wraps up, purification involves low-pressure distillation or recrystallization, depending on the batch size. Some groups try alternative routes using reductive amination methods, but hydrogenation wins most of the time on cost and simplicity.
The backbone of 3,5-dimethylpiperidine supports a wide range of chemical maneuvers. Acylations and alkylations tailor this compound into more complex molecules, while oxidation tweaks the ring, helping chemists introduce hydrophilic groups for solubility shifts. With the lone nitrogen, this piperidine easily forms salts with acids, which get used in pharmaceutical synthesis. Substitutions or metalation at other positions on the ring support medicinal chemistry programs looking for new drug leads or agrochemicals. Real value comes from how easily researchers can slot it into bigger frameworks, thanks to strong ring stability and amenable reactivity.
Across chemical suppliers and literature, this molecule appears under several tags. The major synonyms include "3,5-dimethyl-hexahydropyridine", "NSC 69182", and "Hexahydro-3,5-dimethylpyridine". European chemicals inventories and the American Chemical Society alike prefer “3,5-dimethylpiperidine” for clarity, but catalog numbers also link to "3,5-dimethylpiperidine, technical" or "3,5-dimethylpiperidine, racemic". Searching any of these lands you on the same shelf, just under a different sign.
Handling 3,5-dimethylpiperidine means working with a chemical that releases vapors at room temperature, so folks running syntheses make sure fume hoods pull strong. If it touches your skin, expect local irritation instead of instant burns, but routine use of gloves and safety glasses stays non-negotiable. Lab SOPs cover proper waste pickup, avoiding drains by trapping spills in sand or absorbents before sending off for organic disposal. Fire hazards stay minor, though, since the flash point sits high compared to many small amines. Each drum or bottle comes with up-to-date SDS data, and European REACH rules demand tracking from purchase to waste.
Demand for 3,5-dimethylpiperidine rises in medicinal chemistry, where the compound fits into synthesis schemes for antihistamines, CNS drugs, and enzyme inhibitors. Crop protection chemists draw on the ring skeleton to design new pesticide backbones that resist rapid sunlight breakdown or enzymatic attack. Materials scientists stretch its utility further, using it as a template for specialty polymers where ring structure affects flexibility and thermal resistance. Analytical chemists spike calibration standards with it, tracking behavior of methylated amines in environmental samples. Each field leans in for a different reason, but the main draw sits with its blend of stability and easy reactivity.
The current wave of research focuses on enantioselective synthesis, since demand exists for pure stereoisomers in pharma. Pharmaceutical pipelines explore new piperidine-containing molecules for broader disease targets, and 3,5-dimethylpiperidine forms the starting block for several candidates. Polymer chemistry groups chase after new adhesives and films using its rigid structure. Academic labs examine reaction networks to unlock greener syntheses, reducing waste with alternative hydrogen sources or biocatalysts. Analytical methods improve quantification and impurity tracking, since smaller batch sizes and higher purity demands force tighter analytical standards than in decades past.
Early studies on piperidine analogs set the stage for modern safety assessments, and more recent work uncovers low to moderate acute toxicity for 3,5-dimethylpiperidine. Most animal trials, quoted in regulatory filings, show that lethal doses sit far above what you encounter in a lab setting. Chronic exposure data remain sparse, so industrial users trace vapor levels and keep air concentrations low just in case of unknown long-term effects. In my own work, gloves and goggles stopped minor skin redness from accidental contact, which lines up with published skin and eye irritation data. Broader toxicity studies are still rolling out, especially as agrochemical programs stretch its use profiles.
Researchers aim to extend the groundwork set by this compound, aiming for chiral separations at scale and bio-based feedstocks for greener production. As pharma programs chase selectivity and speed, building blocks like 3,5-dimethylpiperidine offer shortcuts to new chemical space that other tools struggle to match. There’s a strong push for circular chemistry and solvent-free synthesis, and piperidine derivatives will ride that wave as supply chain transparency becomes standard. As a repeat user, I see more demand for custom derivatives and higher purity samples, with suppliers sprinting to catch up with R&D labs on both sides of the Atlantic.
3,5-Dimethylpiperidine doesn’t sound like the sort of thing you drop into a pot of soup, but for chemists, it’s a solid old friend. I’ve had enough brushes with similar molecules to see it pop up where nitrogen-based rings get attention. The chemical formula is C7H17N. Piperidine by itself is a six-membered ring (five carbons, one nitrogen). The "3,5-dimethyl" part tells us that there are methyl groups (simple –CH3 branches) sticking off the third and fifth carbon positions on that ring.
Nothing beats sketching it by hand, but here’s the rundown: picture a hexagon. Replace one of the corners with a nitrogen atom, and tack small lines (the methyl groups) on the third and fifth carbon atoms, counting from the nitrogen. For those who get a kick out of IUPAC-style precision, we’re talking about a six-membered saturated ring, with a –CH3 coming off at spots three and five, giving:
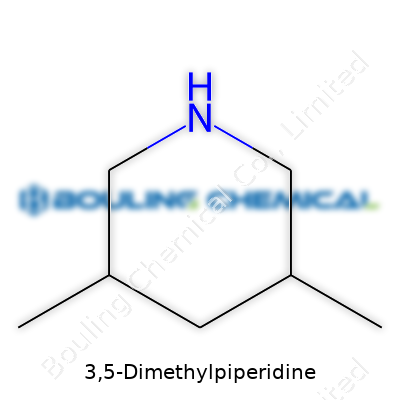
Structure: - A six-membered piperidine ring. - Nitrogen at the first position. - Methyl groups at positions 3 and 5.
If you’ve seen piperidine or its methylated cousins before, this modified structure may seem like a subtle tweak, but even small changes like these punch above their weight in practical chemistry. With the addition of two methyls, the molecular weight bumps up to about 115.22 g/mol. This may look like a mere numbers game, but those methyl groups change properties in important ways—boiling point, solubility, chemical reactivity.
The world never runs out of need for new building blocks, and 3,5-dimethylpiperidine fills a unique niche. Whether it’s for making drug precursors, designing agrochemicals, or simply as an intermediate, slight shifts in a molecule’s structure can make a product safer, more potent, or easier to handle. The basic ring structure is tough and adaptable, but methyl groups can fine-tune it—making molecules stickier, slipperier, or more selective in reactions.
I ran into similar methylated piperidines while working in a lab focused on medicinal chemistry. There, even small tweaks, like popping a methyl group onto a piperidine, completely flip biological activity. 3,5-dimethylpiperidine isn’t just a curiosity: it's a stepping stone for diverse and essential compounds. In pharmaceuticals, it can lead the way to tailored drugs that don’t just work, but work better and with fewer side effects.
Putting together highly specific molecules usually means juggling yields, purity, and cost. For making 3,5-dimethylpiperidine, reliable starting materials and controlled reaction conditions are key. One older route uses hydrogenation of corresponding pyridine derivatives, but modern approaches often look for greener chemistry, leveraging milder reagents, less waste, and better selectivity.
Shifting focus to more efficient synthesis tracks directly to environmental and ethical concerns. Cleaner processes cut hazardous byproducts, lower costs, and offer a better deal to both the operator and the planet. Chemical research keeps pushing toward techniques that use safer solvents and avoid harsh catalysts, with an eye toward both regulatory compliance and real-world practicality.
Anyone in research or industry thinking about 3,5-dimethylpiperidine will find themselves looking not just at its formula or basic structure, but also at the broader context—costs, availability, downstream chemistry, and regulatory pressure. Tools like X-ray crystallography and NMR give clarity about its shape and behavior, while practical experience shapes safer and cleaner ways to handle, store, and transform it.
3,5-Dimethylpiperidine isn’t a name that rolls off the tongue or fills headlines, but ask any chemist who’s waded through complicated syntheses, and they’ll tell you how much this compound matters. Coming across 3,5-dimethylpiperidine in a lab means jumping straight into the world of specialty chemicals, pharmaceutical research, and even material science—places where small tweaks in a molecule can lead to big changes on a global scale.
If you’ve spent hours watching someone draw out synthetic routes for new drugs, you know about building blocks. 3,5-dimethylpiperidine carries those two added methyl groups that shift its properties just enough to turn heads among pharmaceutical chemists. Turning a basic molecule into a piperidine derivative can adjust how drugs interact with our bodies, not just in test tubes. Think about antihistamines, certain antidepressants, and complex anti-cancer drugs—researchers often play with cyclic amines like this one to get a better fit at a biological target. It’s not about creating another clone but about tweaking reactions to deliver improved stability or cut down on nasty side effects.
Years back, I watched as a bench chemist used a dimethylpiperidine derivative as an intermediate while hunting for better treatments for neurological disorders. Instead of dead-ends, those small methyl shifts gave enough wiggle room to bypass some metabolic problems—sometimes, a career can hinge on such a minor change. There’s a satisfying feeling in finding a sweet spot where a reaction actually goes better because of that small substructure.
Not all of 3,5-dimethylpiperidine’s value lives in medicine. In my time consulting for specialty chemicals firms, requests for neat cyclic amines almost always had a purpose—improving processes or giving certain products a tougher backbone. Some polymer chemists like using molecules with hidden flexibility. These piperidines help tune polymers for durability, especially for things exposed to heat or harsh solvents. Its structure allows it to slide into polymer chains as a kind of spacer, changing how those chains interact—the toughness of some coatings and resins owes a lot to these tweaks.
And when the electronics industry needs additives for new materials or advanced adhesives, little molecules like this keep showing up on the ingredients list. You don’t need a room full of it, just a few grams to influence the properties in a big batch of materials. The same goes for agrochemical research. At one point, I saw this compound in test runs to develop more selective herbicides: the goal was higher crop yield and less by-product stuck in the soil.
Every time the molecule comes up, I think about hurdles more than hype. Nobody wants to get choked up on supply chain issues—many intermediates like this still rely on petroleum-based inputs. Moving to bio-based versions or using cleaner synthesis routes could cut waste and lower costs. Stepping up domestic production also keeps prices under control, as reliance on imports usually drives up delays and paperwork headaches.
One way through this maze: collaboration. Chemists and suppliers have started pooling data on greener routes and safer storage methods. Some labs now run pilot plants using recyclables as feedstock, replacing harsher solvents with milder alternatives. If you can make these molecules on demand, close to where they’re needed, it supports both local jobs and global supply resilience.
Real change isn’t about flashy breakthroughs. It’s often in the repeated choices a few thousand people make, adjusting a recipe, upgrading a process, or shuffling priorities toward something sustainable. 3,5-Dimethylpiperidine might not headline the next blockbuster drug or the latest smartphone adhesive, but its role behind the scenes proves how even small molecules can drive big improvements across industries.
Stepping into the world of 3,5-dimethylpiperidine feels a bit like joining a chemistry club full of quirky personalities. Straight out, this compound stands as a six-membered ring, holding its head up with two methyl groups placed at opposite sides — carbons three and five, to be precise. This unique arrangement shapes much of its character, both physically and chemically.
Set this stuff out on the lab bench, and most folks will spot a colorless liquid or, in chillier spots, a lump of white or pale solid. It carries a faint but piercing ammonia-like scent — a reminder that the nitrogen atom tucked in the piperidine ring is never shy. With a boiling point circling 147 to 149°C and a melting point near 35°C, you can nudge it from solid to liquid and back without needing extreme conditions. I’ve handled similar cycloalkyl amines before, and these temperature ranges help when you need to distill or separate small batches in practical organic syntheses.
Dip it in water and 3,5-dimethylpiperidine won’t hesitate to mix in. Like other small-ring amines, hydrogen bonding with water molecules comes easy, so you can get pretty concentrated aqueous solutions out of it. Drop it into most organic solvents — think ethanol, ether, even acetone — and the dissolving game remains strong. Anyone doing extractions or creating new intermediate compounds will appreciate that sort of flexibility.
The chemical side shows plenty of vigor. This molecule behaves just like you’d expect from a secondary amine: basic, eager to snatch up protons, and ready to jump into acylation or alkylation reactions. Over many years in the field, dealing with secondary amines, I’ve found these properties hard to beat when making pharmaceutical building blocks or modifying agrochemical molecules. The methyl groups at positions three and five tweak the electron density and, by making access to the nitrogen a tad trickier, they push reactivity into certain directions. For instance, reactions run a little slower here than with plain piperidine, but the trade-off lies in the selectivity the methyls bring.
Walk into a chemistry prep room, and anyone familiar with dialkylpiperidines will reach for the gloves. This compound, as with most low-molecular-weight amines, can be irritating to skin, eyes, and lungs. Its vapor stings a bit in the air, so you keep the bottle sealed tight and tuck it in a cool, well-ventilated spot. These are small but important details once you’ve seen enough spilled amines turn labeling sessions into eye-watering events.
The methyl groups don’t just influence reactions — they shift the way the molecule interacts with things like rubber stoppers or plastic storage. Anyone prepping solutions for days-long reactions knows to pick their materials with a little care. The right bottle can keep a batch fresh; the wrong one can leach strange flavors into your mix.
Why do folks keep reaching for 3,5-dimethylpiperidine? The answer often lies with its chemical attitude and the way it partners up in forming bigger, more intricate molecules. Medicinal chemists have found it handy for tweaking drugs’ structures, especially if you want to adjust solubility or slide past certain metabolic enzymes. Scanning patents, it’s not hard to spot its ring in antiviral leads or parts of catalysts for making polymers.
From my desk, I see a compound balancing practicality — not too finicky, able to be bottled and shipped — with enough personality to make it interesting in synthesis. The quirks of those methyl groups turn straightforward piperidine chemistry into something a bit more nuanced and open up new routes in the search for effective medicines and materials.
Anyone dealing with chemicals like 3,5-Dimethylpiperidine knows these things aren’t just tokens on a material safety data sheet. A small slip, one bottle forgotten open, and the whole bench becomes a headache—sometimes literally, because this stuff can irritate both the skin and the airways. More than a decade surrounded by jars and beakers taught me there’s no shortcut with volatile organics. The habit of tossing lids right back on containers and inspecting seal integrity before every use has probably saved me some trouble and the lab a few incident reports.
3,5-Dimethylpiperidine isn’t going to scream for attention with bright colors or fumes, but don’t let the plain look fool you. Leaks mean vapor. Vapor means trouble for your lungs and eyes. Direct contact brings on rashes, and spills linger in the air. Reports have linked this kind of exposure to headaches, nausea, and a rough time breathing. Worst-case scenario: mixing up incompatible chemicals by accident and knocking together something even more dangerous, which I’ve seen colleagues nearly do in a rush.
Forget tucking the bottle just anywhere. A cool, dry chemical storage cabinet designed for flammable and volatile organics should be the go-to spot. Keep it away from sunlight and heat; these conditions speed up breakdown and evaporation. I’ve walked into storage rooms where things sat next to acids or oxidizers—a recipe for disaster. Segregating chemicals is not micromanaging; it’s common sense. Quick access labels help too. You grab what you need and move on without guessing—no hunting through mystery containers.
Glass holds up well with 3,5-Dimethylpiperidine. Metal containers can react, and plastic leaches under the wrong conditions. Tightly sealed caps keep fumes inside, and any sign of wear on the lid or neck means it’s time to swap out the bottle. Whenever I notice crust or residue around the top, that container leaves circulation immediately. Proper labeling isn’t bureaucracy; it’s your first line of defense against mistakes. No one wants to open a bottle in a hurry and breathe in something toxic.
Anyone working with this chemical should treat it with healthy respect—no shortcuts. I always grab gloves and goggles, and if there’s a chance of splash, a face shield isn’t overkill. Spills happen, even to the careful. Absorbent pads and backup eyewash keep a small problem from turning ugly. Good ventilation beats every fancy filter out there. I prefer a fume hood every time, even for quick transfers. Handling in the open? Only if you enjoy headaches and complaints about the smell lingering for hours.
Training isn’t just a box to check off. Real stories—like the time a half-sealed cap led to days of complaints about the “mystery odor” in our lab—stick with people far more than generic warnings. Teams run smoother when everyone’s clear on how to respond to a spill or exposure. Keeping the emergency numbers posted, spill kits in sight, and a buddy system for high-risk transfers brings confidence to the work. Safety scales with shared responsibility, not individual heroics.
Simple changes help everyone: routine checks, honest communication, and the occasional audit that actually means something. I’ve watched complacency breed more panic than glass breaking or alarms blaring. The day ends best for everyone if we give chemicals like 3,5-Dimethylpiperidine the careful storage and respectful handling they deserve.
Sitting down at the lab bench with the goal of producing 3,5-Dimethylpiperidine, a few things run through the mind. Safety, efficiency, and getting a usable product at the end always matter—whether you work in an industry lab or a graduate program late at night. The challenge is real: not every route gives the yield or cleanliness you want, and no one likes opening a flask to find a resinous mess. From what I’ve seen, the better road to this compound starts with choosing the right starting materials, making the reaction manageable, and paying as much attention to the purification as the synthesis itself.
Most paths toward 3,5-Dimethylpiperidine draw in 2,6-dimethylpyridine, also called 2,6-lutidine, or a related intermediate. Reductive amination using this pyridine as a backbone offers a reliable approach. In practice, the starting material is taken up in a solvent like ethanol or methanol, then combined with a suitable reducing agent. Catalytic hydrogenation using Raney nickel is one favorite in the literature—something you find even in underfunded academic labs. You run the reaction under a hydrogen atmosphere, usually at room temperature and a few atmospheres of pressure, stirring until the thin solution thickens and TLC confirms a near-complete conversion.
Sometimes, folks use 2,6-hexanedione for building up the ring in a two-step process with ammonia or a primary amine. A straightforward condensation builds the skeleton, and then reduction closes the deal. Sodium borohydride gives better selectivity and fewer unpleasant byproducts for small batches, but scaling up shifts people toward catalytic hydrogenation. It’s not always as cost-friendly if you’re not set up for pressure work, so deciding what equipment you have often shapes your strategy as much as the chemistry itself.
Lab syntheses never give textbook results. Raw batches of 3,5-Dimethylpiperidine carry side products—sometimes leftover pyridine, sometimes over-reduced alkanes or colored tars from degraded intermediates. Distillation under reduced pressure works best for separating the product, as 3,5-Dimethylpiperidine distills in a manageable range and stays clear—contamination shows itself fast in the fraction collector. I once tried skipping distillation, thinking crystallization would do the job, but this didn’t separate isomers and left enough smell in the flask to draw complaints.
Treating the crude product with an acid (usually hydrochloric), then basifying and extracting with ether, knocks down a lot of troublemakers. This freebasing-and-extracting routine works better than column chromatography here, not just due to cost but because piperidines tend to streak up silica and waste time. Washing the organic layer with brine before final drying sharpens up purity. Drying tubes filled with potassium carbonate help keep the final product water-free—water doesn’t just dilute yield, it sometimes drags odor and side-reactions right into purity tests.
Experience teaches you that chasing purity on paper and getting it in vials are two different worlds. Catalytic hydrogenation scores with clean products if you’re set up for it, and the acid-base purification step saves more samples than it ruins. Chemists always keep an eye on waste, cost, and the limits of their facilities—no one wants to run a six-hour hydrogenation for half a milliliter of oil. Keeping a slightly broad boiling cut in distillation balances yield against purity, and a little patience with brine washes gives better NMR spectra in the end.
| Names | |
| Preferred IUPAC name | 3,5-Dimethylpiperidine |
| Other names |
3,5-Dimethyl-1-piperidine 3,5-Lutidine hydride |
| Pronunciation | /ˈθriː,faɪv daɪˈmɛθɪl paɪˈpɛrɪdiːn/ |
| Identifiers | |
| CAS Number | 106-04-7 |
| 3D model (JSmol) | `3D0003094475` |
| Beilstein Reference | 1718736 |
| ChEBI | CHEBI:89235 |
| ChEMBL | CHEMBL16338 |
| ChemSpider | 154540 |
| DrugBank | DB04268 |
| ECHA InfoCard | 100.152.296 |
| EC Number | 214-343-2 |
| Gmelin Reference | 79251 |
| KEGG | C06561 |
| MeSH | D04717 |
| PubChem CID | 11838 |
| RTECS number | EK8775000 |
| UNII | NL8U8U4E7I |
| UN number | UN2371 |
| CompTox Dashboard (EPA) | `DTXSID6020667` |
| Properties | |
| Chemical formula | C7H17N |
| Molar mass | 113.21 g/mol |
| Appearance | Colorless to yellow liquid |
| Odor | amine-like |
| Density | 0.857 g/mL |
| Solubility in water | Slightly soluble |
| log P | 0.98 |
| Vapor pressure | 0.58 mmHg (25°C) |
| Acidity (pKa) | 11.13 |
| Basicity (pKb) | 3.22 |
| Magnetic susceptibility (χ) | -49.9·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.426 |
| Viscosity | 0.91 cP (20°C) |
| Dipole moment | 1.49 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 247.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -105.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4186 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS05 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes severe skin burns and eye damage. |
| Precautionary statements | P210, P260, P280, P301+P312, P305+P351+P338, P308+P311 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Flash point | 63 °C |
| Autoignition temperature | 245 °C |
| Explosive limits | 1.1-7.6% (in air) |
| LD50 (median dose) | LD50 (median dose): 405 mg/kg (rat, oral) |
| NIOSH | NA 3,5-DIMETHYLPIPERIDINE |
| PEL (Permissible) | Not established |
| REL (Recommended) | 5 ppm |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
2,6-Dimethylpiperidine 2,5-Dimethylpiperidine 3,5-Dimethylmorpholine Piperidine 3-Methylpiperidine |