Piperidine-4-carboxamide first came into focus during the surge of interest in heterocyclic chemistry in the twentieth century. Organic chemists dug into the piperidine ring system, searching for derivatives with unique pharmacological or industrial uses. The amide at the fourth position gave rise to countless routes in medicinal chemistry. Over years of research, multiple teams built on early syntheses, refining purification steps, and boosting yield through catalytic improvements. Early academic literature reported laborious column chromatography, but as chemical manufacturing grew, the procedures became more streamlined. Now, the carboxamide variant is referenced in patent filings, pharmacological pipelines, and reagent catalogues, marking its path from a laboratory curiosity to a mainstay in many chemical inventories.
Piperidine-4-carboxamide often arrives as a white or off-white crystalline powder. This compound serves as a building block, lending its structure to drug discovery and product development. It is no secret that its stability and versatility open doors across many fields. The ammonium function on the carboxamide allows for ready chemical modification, so labs take advantage of this site in the hunt for the next therapeutic lead. That practical value keeps it around as more than just a background reagent — it sticks, often at the edge of headlines focused on novel syntheses.
Once in hand, the substance carries a moderate molecular weight and dissolves easily in polar solvents, especially water and methanol. The melting point offers the first real clue for quick identification, usually sitting comfortably above everyday room temperature but well below decomposition. That temperature sweet spot signals fewer risks for runaway reactions in the lab. Structurally, the saturated six-membered piperidine ring brings a certain rigidity that chemists appreciate, as it avoids the tricky reactivity of its aromatic cousins. The amide group at position four creates a reliable site for hydrogen bonding, so it takes part eagerly in supramolecular assemblies and solid-state chemistry. Spectroscopic fingerprints, especially in NMR and IR, come through cleanly and make tracking purity less of a headache compared to certain other backbone-modified analogs.
Every vial, drum, or package of piperidine-4-carboxamide must present a clear profile. Ingredient purities tend to fall in line with pharmaceutical or industrial standards — you will see numbers exceeding 98% for research purposes and sometimes pushing 99.5% for active pharmaceutical ingredient work. Documents typically report melting range, molecular formula (C6H12N2O), molecular weight (128.17 g/mol), and trace impurities with thresholds set by regulatory bodies. Storage advice leans toward cool, dry places, sealed to limit moisture ingress and prevent clumping or hydrolysis. Shipping paperwork must mention hazard codes if amounts trigger regulatory concern, with safety instructions printed in visible ink. Well-maintained documentation means anyone — chemist, technician, or auditor — can quickly understand what sits in front of them.
Laboratories and factories prepare piperidine-4-carboxamide through a straightforward, reproducible route from commercially available starting materials. Many use piperidine, reacting it with a carboxylating agent, then converting the acid group to the carboxamide through dehydration, often with coupling agents like EDC or DCC. Some avoid harsh reagents, picking milder approaches, like enzymatic amidation or acid-chloride free conversion, mainly to manage waste and prevent accidental side reactions. The best processes keep byproduct streams simple, so downstream cleaning need not chew up time or effort. Scale-up from flask to reactor demands tight control of rates — and the exotherm, though mild, still calls for attentive monitoring. Any color changes in intermediate stages tip off the need to adjust parameters. Once yield and repeatability reach robust levels, the procedures make their way into manuals or industry best-practice checklists.
Piperidine-4-carboxamide holds up to many harsh and gentle conditions alike, making it a favored partner in a slew of organic transformations. Chemists employ its nitrogen as a nucleophile, pushing into N-alkylations, acylations, and even cyclization sequences that generate spiro and fused systems. The carboxamide group tolerates metal catalysts, acid chlorides, and oxidizers, provided you plan the steps right. Multistep syntheses often use it as a springboard, transforming either the piperidine core or amide for complexity. Decades of reaction optimization favor its conversion into chiral auxiliaries, peptidomimetics, and specialty ligands for catalysis research. In my own experience, a carefully chosen protecting group, paired with mild conditions, readies the molecule for late-stage functionalization — something many researchers prize for library construction.
Across catalogs, piperidine-4-carboxamide answers to a stack of aliases, which keeps searches lively. Some refer to it directly as 4-piperidinecarboxamide, sometimes by its systematic IUPAC name or even as a variant of nicotinamide when context serves synthetic biology. In high-throughput screening, contracts and databases track it under standardized identifiers such as CAS numbers, boosting traceability across continents and labs. Overheads in procurement meetings usually bounce between documentable synonyms to keep paperwork watertight. Pharmaceutical pipelines and certificates stack both trade and scientific names, ensuring peer reviewers, customs officials, and regulatory agencies all stay on the same page.
Dealing with piperidine-4-carboxamide calls for the same level of attention as any nitrogen-containing compound, though it avoids the explosiveness of high-energy reagents. Standard operating procedures point to gloves, goggles, and ventilated workspace. The dust, powder, or solution brings a risk of irritation for skin, eyes, or airways, so engineering controls and training form the first line of defense. Storage in unlabelled containers, accidental mixing with strong oxidizers, or unchecked heat all score as rookie mistakes. Regulatory standards in major jurisdictions, such as REACH and OSHA, define its handling and disposal, so regular audits smooth the division between bench and compliance staff. Facilities install detailed spill, fire, and first aid protocols, reporting any incidents as part of company-wide safety programs.
Piperidine-4-carboxamide finds steady uptake in drug research, biochemistry, and as a key intermediate in the synthesis of specialty chemicals. Medicinal chemists eye its profile in the search for kinase inhibitors or CNS therapeutics, since the piperidine motif appears in approved drugs and reference molecules alike. Material scientists investigate its solid-state assembly for lithium-ion battery innovation and supramolecular assembly. Chemical engineers draft it into catalyst design and environmental testing kits. Its reactivity brings it to the toolkit in academic discovery and industrial practice. A handful of pioneering groups fold it into peptide mimics, enzyme inhibitors, or probe design for fluorescence-based assays, each iteration pushing boundaries in specific scientific niches. These practical deployments reflect how a small structural tweak in a base molecule can translate into leaps across different sectors.
Investment in research continues to push the limits of what piperidine-4-carboxamide can do. In pharma companies, it shows up in combinatorial libraries, often flagged as a promising fragment for structure-activity relationship studies. Many research teams evolve new synthetic analogues, chasing improvements in bioavailability, metabolic stability, and target selectivity. University groups team up with industry to explore greener synthetic routes, aiming to find renewable feedstocks or lower-energy transformations. Structural biology projects circle back to piperidine-4-carboxamide derivatives when exploring new enzyme pockets. My own work in collaborative projects has shown that minor modifications to this core can shift molecule binding dramatically — a reminder of how foundational structure can influence biological outcome. Patent applications from the last decade point to new uses in diagnostics and as precursors for agrochemical products, broadening the horizon for commercial applicability.
Toxicology studies set strict baselines for safety, addressing oral, dermal, and inhalation routes. In rodent testing, high doses lead to mild to moderate symptoms, mostly reversible after cessation. In standard cell-based assays, the parent molecule demonstrates low cytotoxicity compared to more reactive analogues, offering comfort for handler and downstream user. Environmental impact studies highlight that the amide does not break down into persistent pollutants in typical aquatic conditions, but chemists still log all release metrics for risk assessment. Regulatory oversight bars its use in products marketed to sensitive subpopulations until long-term metabolites are fully mapped. Safety data sheets point to minimal bioaccumulation risk but recommend avoiding long contact with skin or mucosa. Continuous monitoring and periodic review of published reports ensures that risk classification remains current as new studies surface.
The future for piperidine-4-carboxamide looks more promising as research continues to uncover new applications. Advances in machine learning for drug design have started to treat this compound as a scaffold for lead optimization through AI-driven modeling. Organic electronics experts dabble in its use as a template for new polymer designs, promising better conductivity or stability. Green chemistry initiatives explore enzymatic synthesis, targeting lower carbon footprints for mass production. In emerging market economies, manufacturers look for cost-effective scaling routes to make specialty chemicals more accessible without sacrificing purity or safety. As global regulations tighten around product safety, smarter methods for synthesis, waste management, and risk reduction develop in parallel. Academic and industry partnerships now work closer, sharing innovation cycles and data through open-access platforms, keeping the collective scientific community at the edge of what's possible with this key chemical.
Piperidine-4-carboxamide sounds like a chemical you’d only see scribbled on a whiteboard in a research lab, but this little molecule supports some big efforts in science and health. You’ll find it woven into the background of projects that matter: drug discovery, neurological research, and even the creation of new medical therapies. Scientists often highlight these “building block” chemicals, but unless you’ve actually worked at the bench or dug through stacks of journal articles, it’s easy to miss their real-world value.
Pharmaceutical chemists reach for piperidine-4-carboxamide when building what’s known as “scaffolds”—the core structures needed to design new medicines. I remember early in my career watching how skilled chemists use molecules like this one to fine-tune potential drugs for Parkinson’s, epilepsy, and even rare autoimmune diseases. Piperidine rings show up as the backbones in plenty of approved drugs because they offer stability, flexibility, and ways to interact with different biological targets.
If you’ve looked into pipeline compounds at biotech firms, you’ll notice that heterocyclic amides such as piperidine-4-carboxamide often crop up in treatments for depression, schizophrenia, and chronic pain. In some cases, their chemical structure helps produce molecules that bind tightly and selectively to receptors in the brain or immune system. Test results show these attributes can translate into fewer side effects or longer-lasting relief for patients.
I’ve sat in on meetings where researchers debated which molecular modifications could lead to a better candidate for clinical trials. Rather than start from zero, researchers prefer to lean on well-known “core” structures that bring a track record of safety and versatility, like what piperidine-4-carboxamide provides. It also acts as a stepping stone for creating analogs—slightly varied versions of a core drug—which broadens options for tackling challenging diseases.
Peer-reviewed studies show how medicinal chemists take a molecule like piperidine-4-carboxamide, dress it up with different side groups, and check how those changes influence bioactivity. This isn’t just academic tinkering; real progress against antibiotic resistance and treatment-resistant cancers comes from such incremental advances.
The market for specialty chemicals never stays still. Today, sources for piperidine-4-carboxamide range from major chemical suppliers in Europe, the US, and Asia. Safe handling is crucial since, like many lab reagents, improper storage or careless synthesis can spark trouble. I’ve learned that regulatory questions can also slow research—especially if the molecule’s relatives appear on controlled substance lists or need strict tracking.
If teams want to fully tap its potential, more open data sharing helps. Some labs keep their tweaks in-house, which can slow the process of finding out which combinations work best. By publishing negative results, or sharing their synthetic routes, scientists can save each other from waste and dead ends. This open practice can speed up everything from antibiotic discovery to more targeted psychiatric drugs.
We may never see the name piperidine-4-carboxamide in a drugstore advertisement, but its story runs through every successful bench-to-bedside transformation in modern medicine. The more we understand the roles of such key intermediates in pharmaceuticals and medical research, the closer we get to real breakthroughs for people who need them most.
Piperidine-4-Carboxamide pops up regularly in chemistry labs and research facilities. Folks working with it often deal with it as an intermediate while building up other compounds in pharmaceuticals or fine chemicals. Most don’t stumble onto it in daily life, but for chemists, it’s familiar—kind of like a wrench in a mechanic’s toolbox.
I remember the first time I dealt with it back in grad school. In our department, safety went hand-in-hand with respect for the unknown. Even chemicals with clean-sounding names could turn tricky fast. Piperidine derivatives, including Piperidine-4-Carboxamide, may look harmless, but chemistry teaches caution from day one. Before even screwing the cap off a bottle, I checked the Safety Data Sheet (SDS), pulled on gloves, eye protection, and made sure the fume hood was running. My hands still smelled faintly of nitrile by lunchtime, even after washing them twice, but nobody in that lab joked about shortcuts.
Chemical manufacturers classify Piperidine-4-Carboxamide as hazardous mostly because of its structure. Many piperidines can irritate the skin, eyes, and lungs. According to research published in chemical safety journals, exposure to piperidine compounds even at low doses can mean headaches or nausea for some people. Accidental splashes on the skin or eyes lead to irritation and, if inhaled as dust or vapor, throat discomfort or coughing. Detailed animal studies are rare, but with piperidines, experts recommend treating dust and vapors as unsafe until proven otherwise.
In any working chemistry lab, rushing or skipping steps never pays off. The number of researchers who have shared stories about minor accidents—some with lifelong scars—always served as a sober reminder to me. Gloves, goggles, and fume hoods stop most problems before they start. Having a spill kit or eyewash bottle close by isn’t just for show. Quick action limits exposure, and awareness keeps coworkers safe too. I learned early on that anything designed to react—like amides and amines—brings risks even before mixing begins.
No batch of Piperidine-4-Carboxamide belongs in the hands of the untrained. Every responsible lab checks that everyone using chemicals like this knows how to handle spills, knows where the exit is, and keeps first aid within reach. Regular drills, up-to-date SDS binders, and experienced mentors create an environment where emergencies rarely turn into disasters. It’s no exaggeration to say that preparation and teamwork make a safer space for everyone.
Each year brings better gloves, more accurate fume hoods, and a push toward greener chemistry. While some research still needs piperidine derivatives, chemists follow the latest best practices and switch to less hazardous alternatives whenever they can. Universities and companies now reward efforts to lower risk in their methods—sometimes with grants, sometimes with recognition—but always with gratitude from those spending long hours in the lab. More transparent reporting and sharing near-miss stories help everyone, not just those in the same company or country.
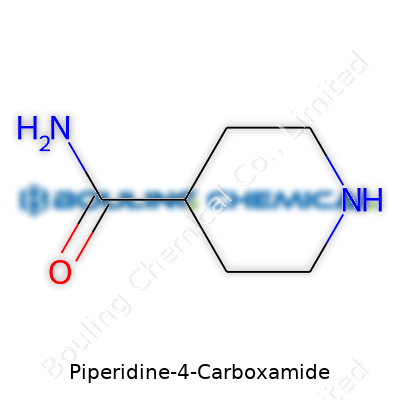
Piperidine-4-carboxamide is one of those chemicals that quickly turns lab conversations into serious science. Its structure starts with a piperidine ring, which chemists recognize as a six-membered ring saturated with nitrogen—that nitrogen gives the molecule special behavior in both synthesis and biological contexts. The “4-carboxamide” piece points to the amide group attached to the fourth position on the ring. Lay out the molecule and you’ll see: five carbon atoms and one nitrogen form the ring; at the fourth carbon, a carboxamide group (–CONH2) branches off.
For drug discovery, knowing this layout means researchers have a foundation for imagining how new medicines interact with the body. The piperidine core appears in everything from pharmaceuticals to industrial chemicals. Add a carboxamide at the right spot, and new bioactivities come to the surface. Medicinal chemists constantly track these details because a single structural change can mean the difference between a safe painkiller and a harmful compound.
In the case of Piperidine-4-carboxamide, the presence of the amide group isn’t just decorative. Amide groups introduce hydrogen bonding interactions, which allow the molecule to stick to proteins or enzymes in a predictable way. For those of us who have ever worked with molecular docking studies, these little connections drive almost everything—binding, selectivity, and eventual effects in a living organism. Piperidine-based structures also tend to resist metabolic breakdown, which can mean longer action for medicines or more persistent chemicals in the environment. There’s responsibility in both cases: chemists design with both benefit and potential risk in mind.
Recently published studies highlight that piperidine-based amides serve as keystones for central nervous system agents, antiviral medications, and even agri-chemicals. Both the European Chemicals Agency and scientific databases point out how structural tweaks around the piperidine ring can lead to new uses. For example, Piperidine-4-carboxamide lays groundwork for derivatives that show anti-inflammatory or neuroprotective effects. Out in the real world, familiar drugs such as paroxetine take advantage of the same ring system, just with differences in functional groups. This kind of structural innovation doesn't just spark curiosity—it underpins patents, drives new research, and nudges the industry forward.
There’s a side to chemicals like Piperidine-4-carboxamide that gets less attention: responsible sourcing and handling. Many raw chemicals end up in legitimate labs and industries, but bad actors sometimes seek these for illicit activities. Industry guidelines and regulations serve a direct purpose here. Laboratories often keep logs of precursor usage and apply checks before ordering. Manufacturers provide material safety data sheets so labs can work safely.
In my experience, smaller research labs sometimes skip steps, not out of malice but because time and budgets run short. That’s risky business. Chemical safety requires discipline, especially when dealing with structures likely to end up in medicines or potent reagents. Training, clear protocols, and regular inventory checks make all the difference. I know colleagues who caught potential misuses early just by double-checking shipments and usage records.
To tap the full value of chemicals like Piperidine-4-carboxamide, communication between research, industry, and regulators needs real focus. Open scientific databases, transparent supply chains, and ongoing safety education keep the benefits flowing while closing the door on problems. The chemical structure isn't just a curiosity—it's a reminder of the power and responsibility that comes with modern chemistry.
Piperidine-4-carboxamide sits on many chemical shelves in labs, research institutes, and production facilities. Keeping it safe and stable isn’t just chemistry—it’s common sense and a responsibility. If storage gets sloppy, quality dives and safety goes out the window. Chemical degradation not only throws off experiments but can bring real risks. Small mistakes in handling or storage keep safety officers and lab workers up at night, because they know the stakes stretch beyond inconvenience—they reach into health, reputation, and legal compliance.
In my years working alongside chemists, I’ve seen simple errors wreck valuable stock. Piperidine-4-carboxamide should always go in an airtight container. Exposure to air leads to slow, creeping decay—sometimes undetectable until results stop making sense. Moisture sneaks in and starts unwanted reactions. Choosing glass over plastic prevents slow leaching that plastic sometimes brings. Lids must fit snugly every time.
Ambient temperature swings change the game. Labs often forget the consequences of seasonal shifts or shared storage rooms. Piperidine-4-carboxamide prefers a cool, stable spot—ideally between 2°C and 8°C. Fridges work best, as regular room temperature leaves it vulnerable to slow chemical changes. Never freeze unless the supplier’s certificate clearly states freezing won’t affect quality. The right temperature is not just about shelf life—it’s about getting reliable results every time you draw from the bottle.
I once watched a colleague struggle after storing a light-sensitive compound near a window; weeks later, their research hit a wall thanks to chemical breakdown. While not all piperidine derivatives fry instantly under sunbeams, practice proves that every compound lasts longer away from direct light. Amber bottles or a drawer help extend the life of your stock.
Cross-contamination ruins more batches than most people realize. I learned this early, helping out in a crowded teaching lab. One mislabeled lid and the next experiment collapsed because trace volumes of other organics ended up as unintended guests. Keep piperidine-4-carboxamide away from acids, bases, and oxidizers. Always label storage spots and bottles clearly, especially if other white powders or crystalline substances are nearby. Never share scoops or spatulas between bottles.
Big companies and universities lean on digital logs and inventory management systems, but hand-written labels and old-school notebooks still matter. Every vial needs a clear arrival date, batch number, supplier, and storage condition written right on the bottle and in a record. Tracking lets you spot trouble before it spreads. If contamination or degradation does appear, it’s easier to find the source. For anyone who’s ever faced an audit, accuracy here also keeps headaches at bay.
Piperidine-4-carboxamide doesn’t top the charts for volatility or hazard, but you never get second chances if spills or mislabeling happen. Always leave it behind locked cabinet doors, away from casual hands and busy foot traffic. Keep spill kits, gloves, and safety sheets (SDS) within arm’s reach. Training new staff each semester or quarter—not just once a year—keeps the standards strong.
Investing attention in the simple habits—cool, dark, dry, labeled, locked away—pays back every day. Science only earns its results when chemicals stay true to spec. That keeps everyone safer, avoids lost dollars, and backs up the credibility of the work. After so many years in labs, I believe good storage suggests good science.
A lot of researchers recognize Piperidine-4-Carboxamide by its structure—a six-membered piperidine ring with a carboxamide group. This might sound technical, but it puts the compound squarely within the chemical families that show real promise for drug discovery and material science. Scientists often look for molecules like this because of the range of experiments they can fuel.
Piperidine-4-Carboxamide keeps showing up on medicinal chemists’ radar thanks to its backbone. This scaffold supports tweaks and modifications, helping researchers build libraries of test compounds. Many modern drugs owe their origins to simple molecules like this, cobbled together and improved upon in the lab. Statins, antihistamines, and antidepressants draw inspiration from similar chemical layouts.
Medicinal chemistry research leans heavily on reliable building blocks. Piperidine-4-Carboxamide lets researchers swap out parts and test what happens. Looking at my own time spent helping graduate students with their synthetic chemistry, some of the most successful molecules started with a small scaffold modified into dozens of different shapes. Sometimes, one tweak surprises everyone—suddenly you’ve got a candidate that interacts with a key protein or skips right through cell membranes. Piperidine-4-Carboxamide joins that toolbox for sure, offering up a path for antipsychotic, analgesic, or antiviral development.
Big drug screens can tell us which compounds show promise against diseases like cancer, Parkinson’s, or malaria. Labs often rely on a stash of carboxamides, since these compounds slot neatly into automated testing platforms for new enzyme or receptor targets. That way, researchers get quick leads about programs worth advancing. One push in drug discovery right now involves targeting stubborn proteins—like those involved in cancer resistance. My experience working alongside screening teams showed me just how important structural diversity is, and Piperidine-4-Carboxamide absolutely helps here.
Researchers working with polymers sometimes reach for piperidine derivatives while searching for new materials with unique properties. Mixing and modifying Piperidine-4-Carboxamide lets them tailor polymers for targeted drug delivery, sensors, or even fast-response coatings. The carboxamide group is friendly to further chemical changes, which matters when designing molecules for surface chemistry or environmental detection. In one of the university labs I worked with, the students synthesized small carboxamides like this to experiment with self-assembling films—fields that cross over with electronics and medicine.
Neurological research has begun to mine carboxamide-containing molecules for effects on neurotransmission. Some piperidine-based compounds have already changed how labs look at dopamine and serotonin receptors. If new derivatives from Piperidine-4-Carboxamide hit the right balance between effectiveness and safety, they could anchor entire projects investigating schizophrenia, pain, or memory disorders. Seeing firsthand how nerve cell cultures react to chemical libraries like this convinced me of their continuing value.
Researchers keep needing practical, versatile molecules. Piperidine-4-Carboxamide shows its strength by letting established labs and small startups alike try new modifications with solid, reproducible chemistry. The compound’s playbook covers medicinal breakthroughs, material design, and biochemistry—areas that don’t stand still. Keeping molecules like this on hand pushes research in the right direction, as fresh questions keep coming up and old ones get new answers.
| Names | |
| Preferred IUPAC name | 4-Piperidinecarboxamide |
| Other names |
4-Piperidinecarboxamide Piperidin-4-carboxamide 4-Carbamoylpiperidine |
| Pronunciation | /paɪˌpɛrɪdiːn.fɔːr.kɑːrˈbɒk.sə.maɪd/ |
| Identifiers | |
| CAS Number | 22919-24-4 |
| 3D model (JSmol) | `3d:JSmol` |
| Beilstein Reference | 1221941 |
| ChEBI | CHEBI:44109 |
| ChEMBL | CHEMBL443142 |
| ChemSpider | 189844 |
| DrugBank | DB08186 |
| ECHA InfoCard | 100_140_2 |
| EC Number | 3.5.1.87 |
| Gmelin Reference | 109211 |
| KEGG | C06355 |
| MeSH | D010875 |
| PubChem CID | 11832341 |
| RTECS number | UG4025000 |
| UNII | H1052G446A |
| UN number | UN2810 |
| CompTox Dashboard (EPA) | DTXSID3058203 |
| Properties | |
| Chemical formula | C6H12N2O |
| Molar mass | 114.16 g/mol |
| Appearance | White to off-white solid |
| Odor | Amine-like |
| Density | 1.06 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | -0.65 |
| Vapor pressure | 0.0227 mmHg at 25°C |
| Acidity (pKa) | 11.2 |
| Basicity (pKb) | 2.88 |
| Magnetic susceptibility (χ) | -56.2·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.486 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.54 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 218.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -520.8 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation |
| GHS labelling | GHS07 |
| Pictograms | HCN1CCC(NC(N)=O)CC1 |
| Signal word | Warning |
| Hazard statements | H302, H314 |
| Precautionary statements | Precautionary statements: P261, P280, P304+P340, P312, P405, P501 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Flash point | > 113.8 °C |
| Autoignition temperature | 355°C |
| Lethal dose or concentration | LD₅₀ Oral (rat) > 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose) Oral Rat: 1550 mg/kg |
| NIOSH | SE1677000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.5 mg |
| Related compounds | |
| Related compounds |
Piperidine Isonipecotic acid N-Methylpiperidine-4-carboxamide Piperidine-4-carboxylic acid Piperidine-4-carbonitrile |