Chemists started paying attention to piperidine-4-carbothioamide back in the middle of the 20th century when the pharmaceutical boom encouraged curiosity about nitrogen and sulfur functionalities. Much of the initial research drew from the broader field of thioamide chemistry, fueled by a demand for tailored intermediates to advance drug synthesis. Labs in Europe experimented with piperidine derivatives hoping to design stronger and safer medicinal agents. Over decades, this compound moved from a basic building block in research settings to a genuine contender in specialty chemistry.
At its core, piperidine-4-carbothioamide presents a six-membered saturated ring featuring both a nitrogen at the 1-position and a thioamide group directly on the fourth carbon. The presence of sulfur introduces a distinct reactivity, which sets it apart from simpler amides. Many chemists in pharmaceuticals or advanced materials refer to it as a “platform” molecule, since swapping out the thioamide or modifying the ring opens doors to unique molecular architectures.
Under standard conditions, it appears as a white to pale yellow crystalline powder, soluble in many polar aprotic solvents. The melting point sits in the modest range (around 120-140°C) and it has a fairly robust shelf life, provided containers are tightly sealed and kept away from moisture. The distinct odor—some describe it as faintly sulfurous—signals the thioamide group. From experience, handling it in the lab highlights the importance of working in a well-ventilated space; its vapors, while not overwhelming, can linger. Its structure enables tautomerism, switching between thione and thiol forms under various pH and temperature conditions, which affects its chemical reactivity.
Producers usually specify purity at 98% or higher for lab-grade samples, while technical grades support broader industrial applications. Safety labeling must reflect the substance’s thioamide core and the exposure limits established by regulatory agencies. Batches often include lot numbers, synthesis dates, and recommended storage conditions—below room temperature, dry, and away from light. Labels also show GHS pictograms, warning about potential irritancy and environmental impact. Reliable suppliers provide certificates of analysis outlining impurities and moisture content, details that researchers trust for repeated experiments.
The most common laboratory route starts with 4-piperidinone, converting it through oximation and thionation. In one setup, students add Lawesson’s reagent or phosphorus pentasulfide to a stirred solution containing the oxime, wisely favoring anhydrous conditions and temperature control to avoid side reactions. Yields run high when the setup remains dry and the reaction doesn’t run hot. After the reaction, extraction and crystallization follow, with cold ethanol as a favored solvent for cleanup. Some teams choose alternative thionating reagents, but the traditional sulfur-rich approach stands out for consistency and scale-up.
This compound welcomes nucleophilic attack at the thioamide carbon, leading to cyclizations or further derivatization. It holds its own in multicomponent coupling, fitting well into Ugi-type reactions or acting as a bidentate ligand for transition-metal catalysis. Experienced chemists exploit its versatility to make antitubercular scaffolds and to design potential enzyme inhibitors. Under mild acid or base catalysis, the thioamide group accommodates alkylation, arylation, or cyclization with adjacent functionalities. With oxidative conditions, it can transform into sulfoxides or sulfones, stepping into material science or agrochemical research.
Researchers may encounter piperidine-4-thiocarboxamide, N-thiocarbonyl-4-piperidine, or 4-piperidinylcarbothioamide as alternative names in literature databases or catalogs. These synonyms reflect different naming conventions but all relate to the same fundamental scafold. Commercial suppliers sometimes list the compound under catalog numbers or as part of broader piperidine-based intermediate series. Clear identification through IUPAC nomenclature and structural formulas avoids confusion, especially during cross-referencing between databases or patent searches.
From direct lab experience, gloves, goggles, and well-fitted lab coats prove essential. Prolonged skin contact can cause irritation, particularly around the thioamide functional group, and some studies point to mild sensitization in animal tests. Proper storage in closed, labeled containers limits the risk of accidental exposure or water uptake, which can compromise stability. Fume hoods make a significant difference, helping to disperse vapors and protect lab staff during weighing or transfer. Waste disposal complies with local environmental regulations, focused on thioamide decomposition and minimizing sulfur runoff.
Medicinal chemists investigate piperidine-4-carbothioamide as a precursor for neurologically active pharmaceuticals and potential pesticides. The unique mix of piperidine’s bioactivity and thioamide’s reactivity opens additional doors in enzyme inhibition research. Material scientists test derivatives for corrosion inhibition, tapping into the compound’s sulfur donation ability. In corporate labs, this molecule feeds into multi-step syntheses aiming for antiviral or anti-inflammatory agents. Its adaptable ring turns up in dyes, specialty coatings, and as an intermediate in heterocyclic assembly lines. The breadth of opportunity comes from its smooth integration into established and novel reaction routes.
Recent years brought renewed interest in derivatives that blend carbothioamide rings with aromatic substituents, chasing new possibilities in CNS drug research. Collaborative efforts between academic and industrial research labs focus on optimizing synthetic routes, using greener reagents and recycling solvents. Some labs explore immobilizing the compound onto solid supports, tailoring slow-release agricultural formulations. The competitive biotech sector invests in libraries of thioamide-bearing piperidines for high-throughput screening, setting up partnerships for patent royalties. Symposiums and review papers continue to chart the expanding network of reactions and outcomes linked to this adaptable intermediate.
Animal studies look for potential carcinogenicity and mutagenicity, comparing piperidine-4-carbothioamide to related rings and thioamide derivatives. Results show moderate acute toxicity, mostly from oral and dermal exposure routes. In high doses, thioamides can disturb thyroid hormone production—for instance, levels above established thresholds disrupt iodine metabolism, as documented in rodent models. Lab practice values this data for risk assessments, especially when scaling up beyond bench chemistry. Chronic exposure studies remain less conclusive, partly because of limited widespread use. Regulatory guidance advocates treating all thioamide intermediates as hazardous unless clear evidence suggests otherwise.
Looking forward, demand for new small-molecule drugs and greener chemical processes pushes this molecule into more research agendas. Advances in automated synthesis and AI-driven molecule design add to the pool of piperidine-based compounds, increasing chances for a breakthrough in targeted medicine or specialty materials. As industrial chemists look for thioamide analogs with selective reactivity, piperidine-4-carbothioamide stands poised for custom modification. Efforts to reduce environmental footprints encourage the development of less toxic derivatives, both in process chemistry and final products. Many expect the next wave of research will involve fine-tuning its properties through smart substituent choices, shifting the molecule from a supporting role to a mainstay in chemistry labs worldwide.
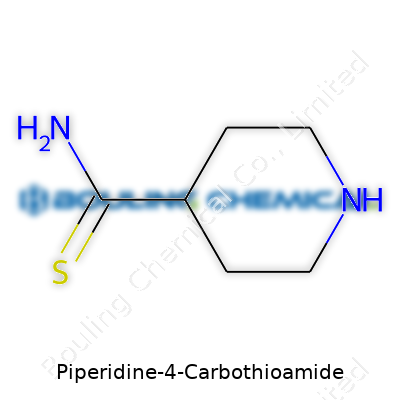
Piperidine-4-carbothioamide belongs to a group of compounds known for containing a distinctive ring, much like the basis for many drugs and chemicals in modern development. At its core, the structure features a six-membered piperidine ring, which is simply a cycle formed from five carbon atoms along with a single nitrogen atom. This nitrogen slot makes the ring less rigid compared to something like benzene, which only carries carbon. The thioamide group, which carries a sulfur atom, attaches at the fourth carbon spot of that ring.
Looking at the chemical formula, you see C6H12N2S. Chemists often sketch this molecule with the piperidine ring drawn as a simple hexagon, open at one point to show the nitrogen. They stick the thioamide group (–CSNH2) right onto the fourth carbon, counting clockwise from that nitrogen. Visualizing these basics, you keep in mind where sulfur sits, since thioamides feature in various chemical reactions thanks to the reactivity of sulfur.
You might spot piperidine-4-carbothioamide in pharmaceutical labs and organic synthesis shops alike. While folks outside chemistry rarely talk about it, this compound helps chemists explore new drug designs. Its ring structure matches up well with molecules in the human body. That sulfur atom in the thioamide group, in particular, lets researchers create bonds that have benefits in medicinal chemistry—potentially leading to pharmaceuticals that fight infections, manage neurological issues, or target certain enzymes.
Back in my lab days, working with various piperidine derivatives felt like opening a toolkit—one molecule, many possible tweaks. When chemists dream up potential blockers for disease-causing proteins or regulators for neurotransmitters, structures like piperidine-4-carbothioamide serve as versatile building blocks. They let you plug new side groups onto the ring or fiddle with the tail, all while keeping that critical nitrogen ready to bind with biological molecules. The compound isn't a frontline prescription drug, but its structure sits quietly in the background, setting the stage for more complex medicines.
With all the benefits, chemicals such as piperidine-4-carbothioamide come with risks. The nitrogen in the ring creates concerns for toxicity if swallowed, inhaled, or touched with bare skin. The thioamide tail doesn't help; sulfur-based groups in organic compounds can sometimes react in ways you don't expect. Safety measures suggest gloves, goggles, and careful attention when transferring or mixing it—even with tiny amounts. Even in college student labs, we learned quickly to double-check the Material Safety Data Sheet.
Accurate labeling, proper ventilation, and strict attention to chemical waste disposal laws cut down the chance of exposure or environmental harm. In the realm of responsible research and industry, these aren't just rules on a page. Every step matters for lab technicians and researchers, especially those new to working with nitrogen or sulfur compounds. I remember a close call with a mislabeled reagent, only caught by a sharp-eyed postdoc. Extra vigilance protects health and the broader ecosystem.
Even as chemists chase after flashier molecules, the foundation built by ring systems like piperidine-4-carbothioamide sticks around. It doesn’t grab headlines, but it supports the ongoing quest for safer, more effective compounds—giving researchers a springboard for smarter drug and material design.
Piperidine-4-carbothioamide would sound like pure jargon to most of us, but this molecule has quietly carved a spot in labs around the globe. In my years talking with pharmaceutical chemists, I learned how quickly research shifts with breakthroughs in compounds like this. Drug developers lean on it not because it’s flashy, but for how it supports the creation of new medicines. Those thioamide groups attached to its piperidine ring open up doors for forming connections with other blocks during drug synthesis. That means easier prototyping, faster results, and sometimes—more hope for tough-to-treat diseases.
Across several journals, researchers report that piperidine-4-carbothioamide can help in building molecules that show genuine promise against cancerous cells. A friend at a major cancer research hospital described screening thousands of variants, where compounds using this scaffold outperformed older options. They look for how these chemicals interact with rogue enzymes or block unwanted signaling. In some hands, versions built with this starting material fought back hard against certain leukemia cell lines and viral infections. With drug resistance continuing to rise, scientists see thioamides like this as fresh ammunition in the toolkit.
Farmers rarely hear about this molecule, but it matters behind the scenes in plant-protection. Chemical companies use piperidine-4-carbothioamide to create test pesticides and fungicides. These efforts help protect staple crops from invasive fungi and insects. At an agricultural tech show last year, a crop scientist showed me data from fields in India where disease-resistant wheat was saved by treatments that started in test tubes with molecules like this. Safe food supplies don’t just happen—they follow painstaking years in research, with molecules like this helping blunt nature’s threats.
Piperidine-4-carbothioamide isn’t just an ingredient. Analytical labs prize it for how reliably it reacts in chemical tests, which helps them improve measurements. Accurate testing sets the stage for food safety, forensic investigations, and even drug purity. Years ago, helping as a lab technician in college, I watched technicians lean on building blocks like this to validate new testing kits. Quick, recognizable reactions mean fewer mistakes and better confidence in results without endless retesting.
The world has woken up to the risk of resistant bacteria and stronger plant diseases. Innovation in molecules, including new classes built with piperidine-4-carbothioamide, can sometimes mean the difference between breakthrough and frustration. To avoid overusing or misapplying new chemicals, regulatory oversight keeps use on track and spots issues early. Better dialogue between research labs, industry players, and regulatory bodies avoids the kind of misuse that gets compounds withdrawn or raises health concerns. Responsible collaboration keeps good science from turning into tomorrow’s scandal—something we all have a stake in.
Piperidine-4-carbothioamide rarely draws headlines, but its fingerprints show up across medicine, agriculture, and testing. As society pushes for safer drugs, fresher food, and cleaner analysis, quietly helpful players like this will keep playing a part. Science isn’t always glamorous, but it sure is vital.
Walking into a chemistry supply catalog, a synthetic chemist gets used to assessing purity claims with a cautious eye. Piperidine-4-carbothioamide’s reported purity often ranges from 95% to 99% in commercial samples. Most common suppliers disclose their batch quality on certificates of analysis, but a glossy label does not always tell the entire story. Trace impurities influence lab outcomes, industrial synthesis, and safety during handling.
Researchers, especially those tackling sensitive pharmaceutical syntheses, worry about more than the headline percentage. An “analytical standard” with 98% purity may meet basic needs in general organics labs. In any drug development cycle, though, overlooked contaminants can spell false leads and expensive failures. A single percent of impurity can introduce unreactive byproducts, sabotage downstream reactions, and muddy spectral data.
Commercial piperidine-4-carbothioamide typically gets produced by acetylation or thionation of piperidine-4-carboxamide. Manufacturing scale plus transportation and storage create different impurity profiles. Some samples harbor starting materials or degradation products. Organosulfur compounds, in particular, can generate unpleasant or hazardous side effects when accumulated, so proper documentation matters.
Companies like Sigma-Aldrich or Alfa Aesar list 97-99% for this compound, confirmed by HPLC, NMR, and sometimes mass spectrometry. Analytical chemists often double-check with their own TLC plates or GC-MS to spot inconsistencies. In my own lab, side-by-side analysis with authentic material saved weeks of troubleshooting. Price tags rarely account for the extra hours spent chasing ghost peaks, which cost more than upfront investment in a better option.
Small-scale suppliers might not track residual solvents, water, or even unidentified byproducts. What seems insignificant at a few hundred micromolars could disrupt enzyme assays or polymerizations. Purity, in this context, draws a line between academic curiosity and reliable, reproducible results.
Some labs accept manufacturer claims at face value, trusting that “research grade” meets their bar. I learned the price of shortcuts during a fellowship, where a key coupling step in a synthetic sequence repeatedly stalled. Only by resourcing freshly certified, high-purity material did the stalled yields turn into high conversion. Without double-checking, years of labor might dissolve into error chasing and blaming “bad luck.”
Choosing chemical reagents boils down to much more than numbers. Industry experts put reputation first, cross-referencing supplier data with independent reviews and peer discussions. Some leading labs even demand third-party analysis on critical lots. Pharmaceutical and biotech companies invest in analytical validation to meet not just productivity targets, but strict regulatory thresholds ensuring patient safety. Researchers support decisions with empirical data, scientific training, and a healthy skepticism that keeps everyone honest.
More stringent quality control stands as one clear solution. Certification should include not just headline purity, but a detailed breakdown of all detectable impurities, solvent levels, and even batch-specific differences. Transparency on manufacturing conditions and storage would let buyers make informed choices. Collaborative networks among scientists help surface hidden issues, creating feedback loops that drive quality upgrades.
Ultimately, purity shapes the success or failure of laboratory and industrial projects. Expectation and vigilance, paired with real-world experience, guide buyers toward better outcomes—and away from the headaches of compromised chemistry.
Piperidine-4-carbothioamide stands out in labs and industry settings, popping up in pharmaceutical and chemical research. Ever since my first job working in early-phase drug synthesis, I learned pretty quickly that mishandling these sorts of compounds doesn’t just create a mess on your lab bench — it can threaten safety, research budgets, and even reputations. Some chemicals just demand respect, and this one lands on that list.
Accidents with specialty chemicals have triggered stricter national and workplace regulations. Reported incidents from the past decade stress the link between poor storage and health issues, not just for trained chemists, but for any person sharing the space. For example, exposure to dusty containers or leaky vials has sent more than one technician to the clinic. Piperidine derivatives, in particular, catch the attention of monitoring agencies like OSHA and European safety boards because of their toxicity, especially if inhaled or absorbed by skin.
Instead of chasing best-case scenarios, people and teams turn to specific, proven steps for keeping Piperidine-4-Carbothioamide safe. In practice, this means keeping the chemical inside a tightly sealed container, using materials resistant to strong chemicals and moisture, such as high-quality glass or compatible plastics. I remember an old colleague’s story: storing a thioamide near a leaky sink became a costly error after humidity seeped into the container. That batch lost integrity, caused confusion in assay results, and led to lost hours.
Laboratories with solid safety reputations focus on temperature control. Keeping storage spaces cool, often between 2–8°C, slows down any unexpected reactions. It helps chemicals like this keep their structure and potency for as long as possible. Regular room temperatures, on the other hand, add risk, especially if the seasons bring steamy summers. It doesn't hurt to slap labels with storage instructions on every bottle—new staff and sleepy researchers alike appreciate that backup.
Letting small mistakes grow can bring heavy consequences. A careless approach leads to ruined experiments, rising costs, plus greater health risks. Industry reports document contamination and accidental exposure most often connect back to rushed storage habits or poor training. Taking Piperidine-4-carbothioamide seriously means thinking about airflow too. Ventilated cabinets, especially those not shared with acids or strong bases, make a clear difference. Cross-contamination brings trouble—sometimes you won’t discover impacts until a crucial project falls apart.
Safety protocols recommend keeping absorbent materials and spill kits nearby, just in case of accidents. Easy-to-read safety data sheets help by listing warning signs and emergency steps, reducing confusion if something spills or leaks. It pays off to do annual checks—throw out expired bottles, review storage policies with new staff, and keep a logbook tracking who last handled what compound.
Many teams already invest in locked chemical cabinets with humidity controls. These aren't just for show. Security and environmental stability support both product quality and researcher health. Science doesn’t need catastrophic reminders to keep it careful—a little planning and smart habits serve well. My own time working in the field taught me that every minute spent updating storage guidelines returns hours of saved trouble down the line.
Most people outside a lab don’t run into piperidine-4-carbothioamide. For anyone who does, safety becomes the main question. This chemical sits among countless compounds researchers and manufacturers use to tinker with pharmaceuticals and specialty drugs. Before speaking about its risks, it helps to remember that chemicals aren’t good or evil — it’s what happens after opening the bottle and how someone treats it that matters.
I’ve read a fair share of studies and safety sheets during years in science writing. Material safety data for piperidine-4-carbothioamide usually lacks long case histories. That’s common for niche or intermediate compounds. Still, experts flag several basic concerns for related compounds: skin and eye irritation, respiratory effects, and possible organ stress during prolonged or repeated contact.
Reports from chemical suppliers and laboratory safety groups describe this compound as an “irritant.” Drop it on your skin or splash it near your face, and you risk inflammation, pain, or short-term itchiness. Swallowing or inhaling, especially in powdered or aerosolized form, creates more problems. Nothing points toward it being wildly more dangerous than many lab chemicals, but lax handling raises the risk for accidents.
Working in labs during grad school, I paid close attention to hazard symbols. Kits and bottles marked with warning diamonds always got more space and better personal protection. Piperidine-based compounds, by their chemical structure, often signal a need for gloves, goggles and, if working with open powders, masks or proper ventilation.
The structure gives hints: anything with a thioamide group can have reactive characteristics, joining with other molecules in ways that sometimes release smelly or potentially harmful gases. Piperidine rings bring some volatility and a risk of inhalation if not handled inside a fume hood.
Major regulatory authorities like the European Chemicals Agency list piperidine derivatives as substances of concern, mainly because so little human data exist and because animal testing reveals signs of irritation, drowsiness or nausea. Many compounds in this family reach the EPA’s hazardous substance registry, mostly out of caution.
Accidents involving similar chemicals, if not cleaned quickly, sometimes cause chemical burns or long-standing respiratory complaints. No reputable source claims piperidine-4-carbothioamide belongs with the most acutely toxic substances, yet it should never be handled like table salt.
Every chemical should get the respect it deserves. Core steps cut down on risk. Those working with this compound should always wear gloves, protective eyewear and coats, use chemical fume hoods and avoid all eating or drinking around open bottles. Training for spills, clear waste disposal plans, and detailed safety instruction for new lab members go farther than any sticker or regulation.
Manufacturers and lab managers can set the tone. Excellent hazard communication—labeling, up-to-date digital safety sheets, and easy reporting of mishaps—keeps everyone on the same page. This approach not only avoids health risks but also builds trust within research and technical fields.
Whenever possible, green chemistry methods encourage scientists to swap out hazardous components for less harmful ones. While not always practical for every synthesis, a long-term commitment to safer alternatives protects both workers and the environment. Transparency in chemical sourcing and clearer reporting will improve understanding of these risks over time, benefiting everyone involved in science and industry.
| Names | |
| Preferred IUPAC name | 4-Piperidinecarbothioamide |
| Other names |
4-Piperidinethioamide Piperidin-4-ylcarbothioamide |
| Pronunciation | /paɪˈpɛrɪdiːn fɔːr kɑːˈbɒθiəˌmaɪd/ |
| Identifiers | |
| CAS Number | 4461-30-1 |
| 3D model (JSmol) | `3d:JSmol` string for **Piperidine-4-Carbothioamide**: ``` NC(=S)N1CCC(CC1) ``` |
| Beilstein Reference | 1264763 |
| ChEBI | CHEBI:18878 |
| ChEMBL | CHEMBL443238 |
| ChemSpider | 21585911 |
| DrugBank | DB08419 |
| ECHA InfoCard | 27a3e889-414b-4506-bbc7-6568fed66787 |
| EC Number | 87121-42-6 |
| Gmelin Reference | 82646 |
| KEGG | C10177 |
| MeSH | D010883 |
| PubChem CID | 16659 |
| RTECS number | TN3150000 |
| UNII | 8I6IXS671J |
| UN number | UN3432 |
| CompTox Dashboard (EPA) | DTXSID6074139 |
| Properties | |
| Chemical formula | C6H12N2S |
| Molar mass | 130.22 g/mol |
| Appearance | White to Off-White Solid |
| Odor | Amine-like |
| Density | 1.1 g/cm³ |
| Solubility in water | Slightly soluble in water |
| log P | 1.11 |
| Vapor pressure | 0.04 mmHg (25°C) |
| Acidity (pKa) | pKa = 11.2 |
| Basicity (pKb) | 3.30 |
| Magnetic susceptibility (χ) | -63.84 × 10⁻⁶ cm³/mol |
| Dipole moment | 4.54 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 229.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -382.5 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation |
| GHS labelling | GHS07, GHS08 |
| Pictograms | NC(=S)C1CCNCC1 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | Precautionary statements: P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P312, P321, P332+P313, P333+P313, P362+P364, P501 |
| Flash point | > 141.8 °C |
| Lethal dose or concentration | LD50 oral rat 590 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 178 mg/kg |
| NIOSH | NIOSH Registry Number: SN8524500 |
| PEL (Permissible) | No specific PEL established. |
| REL (Recommended) | 100-500 mg |
| IDLH (Immediate danger) | N/D |
| Related compounds | |
| Related compounds |
Piperidine Piperidine-4-carboxamide Piperidine-4-carboxylic acid Piperidine-4-carbonitrile Piperidine-4-thiol Piperidine-4-carbaldehyde Piperidine-4-carboxylate |