Back in the mid-20th century, laboratories across Europe chased new ways to fine-tune the structure of simple heterocycles. Out of that rush came piperidine-2-methylamine, an organic building block first noticed more as a curiosity than a tool. Chemists with a background in alkaloid synthesis appreciated the versatility of piperidine rings. Technical journals from the 1970s and 80s mention the molecule as a minor synthetic step, rarely giving it a starring role. Over time, as drug development turned to more tailored pharmaceutical scaffolds, researchers started to pay more attention to compact amines and their substitution patterns, catching on to the potential locked in the 2-methylamine substitution. As the years rolled by, renewed interest in chiral auxiliaries and specialty ligands shined a brighter light on the molecule’s reactivity and functional possibilities.
What stands out about piperidine-2-methylamine is its versatility. Used by pharmacologists, agrochemical researchers, and synthetic chemists alike, this compound’s compact structure blends stability and reactivity—qualities that allow it to serve as a starting point or intermediate in more elaborate syntheses. Drug discovery teams value its role in constructing central nervous system agents, while materials scientists sometimes use it for tuning polymer properties. Instead of being an obscure tool for specialists, piperidine-2-methylamine has become a go-to building block in chemical supply catalogs. It’s easy to order in small research quantities, and labs frequently keep a sample for those projects calling for ring-nitrogen with a little extra punch at the 2-position.
Piperidine-2-methylamine appears as a colorless to pale yellow liquid, though some grades may be sold in crystalline form depending on storage and temperature. It gives off a strong amine odor that reminds anyone who has spent much time in an undergraduate organic lab of wet cleaning rags and sharp chemical notes. The compound dissolves well in water and polar organic solvents, making it easy to handle for most bench procedures. Structurally, it consists of a piperidine ring substituted with a methylamine group at the second carbon, delivering both nucleophilic and basic character. The amine function picks up protons rapidly, and the ring keeps the molecule from reacting with itself, so it stores well and stays stable under typical laboratory conditions.
Manufacturers selling this molecule label their bottles clearly, usually offering CAS number, chemical formula (C6H14N2), molecular weight, and purity—often 95% or higher for research use. Some labs may ask for anhydrous variants or stabilized formulas if water sensitivity becomes an issue in their syntheses. The major suppliers all provide safety data sheets covering potential hazards and storage recommendations, and for good reason. Accurate labeling—a must for safety and regulatory reasons—also gives synthetic chemists the details they need to prepare exact batch protocols, particularly in pharmaceutical or food chemistry settings, where trace impurities can undermine entire projects. Digital inventories have moved beyond ink-on-paper; barcoding and QR codes now help in tracking shipments and usage logs, streamlining operations for both suppliers and buyers.
Lab preparation usually starts with commercially available piperidine, which reacts with suitable methylation reagents to introduce the methylamine group at the 2-position. Reductive amination techniques, often employing formaldehyde and hydrogen donors like sodium cyanoborohydride, offer high yields and limited byproducts. Some industrial syntheses use catalytic hydrogenation methods for better atom economy. Experienced chemists watch for over-alkylation, which can reduce yields or generate troublesome side products, especially in multistep synthesis plans. The trick is to maintain gentle conditions and steady pH levels throughout the process, which helps to keep both selectivity and safety in check.
The 2-methylamine group opens the compound up to all sorts of transformations: acylation, sulfonylation, and cyclization reactions carry the potential for tailored molecules with enhanced properties. Medicinal chemists love using piperidine-2-methylamine in cross-coupling reactions, attaching bioactive moieties or fluorescent tags for tracking inside biological systems. The basic nitrogen can serve as a nucleophile in alkylation reactions while the molecule’s ring system stabilizes against unwanted rearrangement. In my experience, modifying this backbone often proves less finicky than similar transformations on open-chain amines, partly thanks to the protection offered by the piperidine ring. Researchers exploring peptide mimetics or small-molecule ligands value this stability, which lowers the risk of side reactions and waste.
Chemists call this compound by a range of names, depending on their training and the catalog in hand: 2-methylaminopiperidine, piperidin-2-ylmethylamine, and 2-(aminomethyl)piperidine all trace back to the same scaffold. Some companies list it under specific research code numbers for proprietary stock, so it sometimes pays off to double-check structure diagrams. This mix of synonyms occasionally confuses new researchers digging through literature or supplier inventories. The broader chemical community—especially industrial buyers—pushes for tighter naming standards to minimize accidents and mix-ups, a goal that echoes across regulatory and safety bodies worldwide.
Handling piperidine-2-methylamine needs a disciplined approach. The compound’s volatility contributes to inhalation hazards, and its basicity can cause notable skin or eye irritation. Well-aired hoods, gloves, and full eye protection form the bedrock of every protocol in labs handling this sample. Documents like Safety Data Sheets don’t leave room for guesswork: they chart out everything from fire risks—amines can ignite in the right fuel/air mixtures—to emergency measures in event of a spill. Larger-scale manufacturing or commercial use may trigger local and international regulatory requirements covering labeling, waste disposal, and chemical storage. Equipment checks and regular safety training go beyond box-ticking; I’ve seen well-equipped synthetic teams run drills, ensuring swift, informed responses to real-world accidents. These steps pull from regional standards developed over decades. In the United States, OSHA regulations outline workplace exposure limits and personal protective protocols, while the European Union expects REACH-compliant handling and strict record management.
The real-world uses for piperidine-2-methylamine stretch beyond basic research. Pharmaceutical companies tap into the molecule’s structure when looking to fine-tune activity in neurological drug leads. Agrochemical developers use it to test herbicide and insecticide frameworks that aim for greater selectivity and reduced off-target risks. Specialty chemical and materials manufacturers sometimes spot its value in surface chemistry tweaks, adding new properties to polymer chains or molecular coatings. I’ve heard from colleagues in analytical chemistry who use variations of this compound as calibration standards, because its unique spectral signature allows easy identification amid complex mixtures. In academic circles, it often enters the spotlight as a test substrate—its straightforward structure eases the burden on students learning the ropes of modern synthetic chemistry.
Across scientific journals, references to piperidine-2-methylamine have risen steadily as interest in specialty amines grows. Pharmaceutical R&D units in North America, Europe, and Japan pursue new CNS drug scaffolds, and this compound makes frequent appearances in patent filings through the early 2020s. Teams focused on asymmetric catalysis have played with chiral versions of the molecule, hoping for more selective drug profiles and easier separations. For researchers in academic labs, new synthetic methods often feature this compound both as a model system and as a test substrate for broader advances. The cross-pollination of ideas coming from synthesis, analysis, and property testing pushes forward fundamental knowledge and turns basic molecules into practical products faster than ever before.
Toxicologists know that small, nitrogen-rich cycloalkanes deserve cautious respect. Direct research on piperidine-2-methylamine paints a familiar picture: acute inhalation or skin exposure causes irritation, and ingestion triggers nausea or more severe symptoms at higher doses. Metabolic studies in rodents show rapid clearance, mostly through hepatic pathways, though data on chronic low-level exposure remain limited and sometimes contradictory. Large-scale screening projects—especially those tied to regulatory filings—demand detailed tracking of all downstream health endpoints, including reproductive and developmental impact, though no alarming red flags have emerged so far from the controlled lab data published to date. Still, most commercial and academic users treat the compound like other low-molecular-weight amines: with gloves on, fume hoods ready, and eye-wash stations checked.
Looking at the emerging science, piperidine-2-methylamine stays in the running as a core building block for medicinal chemistry, functional polymer design, and analytical chemistry protocols. Research into ring-substituted piperidines and their derivatives promises new CNS-active compounds, and environmental chemists eye structural modifications for greener synthesis. The toolkit for making chiral versions and controlling side reactions grows broader by the year, and with machine learning entering the synthetic planning stage, researchers spot new applications for this broadly useful molecule. Market demand for specialty building blocks shows no sign of slowing, and scientists in rapidly evolving fields like targeted drug delivery or advanced diagnostics keep coming back to the reliable reactivity packed into modest molecules like piperidine-2-methylamine.
Piperidine-2-methylamine isn’t a household name, yet labs and chemical manufacturers know it well. It acts as a handy building block for creating lots of different molecules. Think about a craftsman working with quality lumber; the right starting piece opens up patterns for furniture, homes, even art. In chemistry, compounds like this one give researchers the same creative flexibility. I remember long days in university labs where finding a source of reliable, well-characterized raw material sped up every project and cut down dead ends.
One key use: drug discovery. Researchers often choose Piperidine-2-methylamine to build new molecules aimed at treating illness. For many, this means working to create medicines for conditions as tough as cancer or neurological disorders. Journals reference this compound as a core unit in crafting enzyme inhibitors and experimental therapies. For every new drug, it takes trial and error. Each piece of the molecule must fit, so precision counts. Using established intermediates lets researchers solve the puzzle with fewer unknowns.
The same material helps agriculture, too. Agricultural chemists use it to develop crop protection agents that control pests or fungal outbreaks. A well-designed pesticide or herbicide can mean the difference between harvest success and financial loss. Thanks to the versatility of its structure, Piperidine-2-methylamine lets chemists steer the chemical process toward safer and more effective products. Each discovery gets tested through cycles of greenhouse and field trials—not all succeed, but the better the starting point, the stronger the chance to help feed the world.
Purity matters at every step. Contamination in chemicals used early in manufacturing can spoil whole batches of finished product. In my own lab days, even a minor impurity would set off a chain reaction, wasting hours or days of work. Producers must lean on strong quality controls to deliver consistently pure batches. That’s what sets apart dependable chemical partners from those who cut corners—and what ultimately protects both research integrity and public health.
Of course, such building blocks aren’t just a boon. There’s always the risk that materials like Piperidine-2-methylamine could find their way to illicit enterprises. Drug enforcement agencies watch chemicals like these, knowing that skill alone doesn’t guarantee responsible use. Responsible manufacturers comply with regulations and tracking tools, making sure material goes where it’s supposed to. The balance always tips toward openness and safeguarding both innovation and public safety.
If the world wants smarter, safer treatments and more reliable food production, chemistry isn’t going away. The real challenge circles back to transparency. Sharing data about sourcing, purity, and results builds trust. Partnering with reputable suppliers and upholding standards not only improves output, but also opens doors for the next wave of critical discoveries. Without trust in the building blocks, all science slows down. Investing in quality, traceability, and communication keeps progress on track.
Piperidine-2-methylamine plays a role in chemical research and synthesis. Chemists use it as a building block. Its structure might look simple to anyone familiar with organic chemistry, but anyone handling it should approach it with care.
According to data from agencies like the European Chemicals Agency (ECHA), this compound can irritate skin, eyes, and the respiratory system. If splashed, it feels uncomfortable and can cause lasting burning. Inhaling the vapors for too long may bring headaches, nausea, or dizziness. At higher exposures, some people have fainted or developed more severe symptoms. The risks rise even more if someone drinks or ingests it. Anyone working with it in labs can’t rely on common sense alone—the material safety data sheet gives guidance many take seriously because it's based on real-world exposures and clean-up incidents.
Not every chemical in the lab threatens your life, but ignoring the hazard signs isn’t smart either. Most modern chemistry labs teach workers how to suit up, read warning labels, and wash hands or skin after handling substances like piperidine-2-methylamine. Even short-term carelessness turns into skin rashes or an asthma attack for someone sensitive. Long-term exposure might affect the liver or kidneys, though major health authorities still don’t have large-scale studies just for this one amine. Still, its relatives with similar structures raise enough concern.
Risk grows fast outside a lab. Pouring leftover chemicals down the drain or letting vapors build up in a poorly ventilated room shows where problems start. Piperidine-2-methylamine’s vapors might spread quickly in a warm room, and since our noses tend not to pick up mild smells, people sometimes walk into an unsafe cloud before they realize. I’ve seen folks underestimate the fumes, thinking everything seems fine—until red, watery eyes and tight throats force everyone to clear out.
In my own university lab days, simple routines made a difference. Always working under a fume hood, making sure gloves and goggles fit, keeping containers closed, and never reaching for chemicals in a hurry—all these steps cut down the risk. Most spills and splashes happen because someone gets distracted. The toughest lesson came after a careless moment with a broken vial: quick flushing with water helped, but a chemical burn stuck around to remind me for days.
Teams need training, but it’s also about experience. Having a seasoned lab buddy watch over keeps younger workers from making preventable mistakes. Containment, proper waste disposal, and labeling keep the accidents away. Access control matters: don’t hand over keys or supply piperidine-2-methylamine to someone with no background in handling such substances. These practices pull straight from recommendations by both OSHA and REACH regulations.
Even with precautions, I’ve seen that accidents still happen if people don’t respect the risks. It’s not paranoia; it’s paying attention to years of chemical safety research and reporting. Better labeling and digital tracking make a difference. Finding safer alternatives matters, especially in teaching labs. In industry, substituting less toxic amines where possible could cut both acute and long-term risks. Advocacy for safety equipment and ongoing training keeps the workspace safer—not just for seasoned chemists, but for the new hands following after.
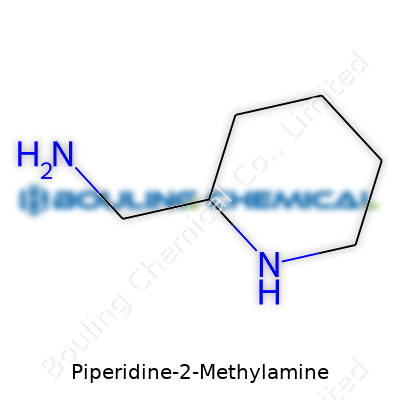
A lot of folks might picture long, complicated chemical models when they hear something like Piperidine-2-Methylamine. The truth is, this compound has a structure that’s fairly straightforward for anyone familiar with basic organic chemistry. At its core sits a six-membered ring, which chemists call a piperidine ring. Imagine a hexagon made mostly of carbon atoms, but with one of those swapped out for a nitrogen atom. This ring looks a bit like a flattened tire, flexible enough to let other groups attach easily. The special twist in Piperidine-2-Methylamine comes from a methylamine group—think of it as a short side chain built from one carbon and a couple of hydrogens, ending in an extra nitrogen. In this molecule, the methylamine hooks up with the second carbon in the piperidine ring.
The shorthand way to write it goes like this: C6H14N2. It’s sometimes easier to picture what this means if you’ve spent time in a lab sketching out molecules on paper. Growing up, I always tried to connect the dots: every corner, a carbon or nitrogen. That helped make sense of what these shapes mean for the way molecules behave.
The chemical backbone isn’t just for show. Small tweaks—like swapping out which carbon the methylamine attaches to—make a big difference in how chemists can use this molecule. Pharmaceutical labs have leaned on ring structures like this for decades because they’re stable, but still open doors for creative chemistry. That six-membered ring anchors the molecule, keeping it from breaking down easily. The amine pieces add flexibility. They can form bonds with acids or other fun chemicals, making Piperidine-2-Methylamine valuable for drug creation or the search for new materials.
It’s easy to overlook the day-to-day uses of molecules like these. Many of our high-performance drugs and even specialty plastics draw from the chemistry that comes out of these simple shapes—proof that the basics stick around because they work.
Experts in chemistry have published loads of studies on piperidine derivatives over the last fifty years. PubChem, a trusted resource in chemical safety and reference, describes structures like Piperidine-2-Methylamine as critical starting points for new medicines. Piperidine frameworks show up in antihistamines, antidepressants, and even in anti-cancer research. One review published by the European Journal of Medicinal Chemistry calls piperidine motifs “privileged structures” in drug discovery—the kind of thing researchers keep coming back to because it works in so many places.
That ring isn’t just a piece of academic trivia. Pharmaceutical companies find ways to spin off new variations because the right positioning of attachments changes how a molecule interacts with the body. Medical research, especially where it comes to treating complex neurological conditions, depends on such tried-and-true chemical cores.
Innovation doesn’t just happen at the whiteboard. Universities and companies alike need to invest in creative chemistry to solve big problems with molecules like Piperidine-2-Methylamine. Educators can make a difference by helping chemistry students master the basics—drawing ring structures, visualizing how side chains can change a molecule’s activity, and connecting all this to real-world lab work. That hands-on experience will help a new generation tackle drug resistance, sustainability in manufacturing, and even safe handling in the workplace.
Chemists who get comfortable with ring structures like piperidines will find themselves prepared for the next rounds of innovation, whether that’s designing smarter treatments or greener ways to make them. Getting the foundations right—down to each bond and attachment—paves the way for bigger breakthroughs.
Anyone who has worked with specialty amines knows their quirks. Piperidine-2-Methylamine stands out for its tendency to produce intense odors and its reputation for volatility. It’s a building block in pharma chemistry and a regular guest in research labs. It’s not the sort of substance to toss on a shelf and forget—one careless step with an amine like this can ruin a whole day’s work, or worse.
Temperatures inside a regular room often swing way more than people think. Even if it seems mild, these shifts trigger changes in volatile liquids such as Piperidine-2-Methylamine. Most labs where I’ve worked keep similar chemicals tucked away in tight-sealing bottles made from amber glass. Glass doesn’t react, and the dark color blocks out light, keeping breakdowns to a minimum.
I once saw a clear bottle left in the sun—its contents turned yellow and started to smell much sharper. Light and heat turn this compound into a headache in more ways than one. A refrigerator—meaning a dedicated, spark-proof chemical fridge, not the lunchroom kind—keeps the temperature steady. On days when the central air in the building failed, the cold storage staff always seemed far less flustered than those keeping chemicals at room temp.
Moisture seeps into poorly sealed bottles fast. Piperidine-2-Methylamine is like a sponge for water in the air, and in my own experience, a bottle exposed to humidity will discolor or even form weird residue in the cap. Once that happens, the accuracy of any experiment takes a hit. Avoiding this means storing the bottle tightly closed and maybe even tossing in a desiccant pack with other sensitive reagents. These simple steps limit corrosion, keep purity high, and prevent contamination.
Back in school, I watched a colleague open something unlabeled, assuming it was harmless—turns out it was an amine. A quick dash to the eyewash station, plus a ruined experiment. All chemicals deserve careful labeling, but volatile amines demand clear, permanent markings. Write out the full chemical name, not just a code. Expiry dates come in handy; even sealed reagents have a shelf life.
Piperidine-2-Methylamine vapors spread fast. Even a minor spill makes a hood reek for hours. Storing anything with flammable vapor away from ignition sources lowers risk dramatically. Dedicated flammables cabinets lined with metal, sitting far from heat or sunlight, keep things safe. The real game-changer is ventilation—airflow keeps trace fumes from settling, something everyone in the lab appreciates. I always check for the nearest spill kit, because it’s better to spend five minutes reviewing emergency gear in advance than scramble during an actual mess.
Rules exist for a reason. Piperidine-2-Methylamine sits on regulatory watch lists in many countries since it plays a role in both the legitimate and darker corners of chemistry. Track every purchase, log every use, and follow both local and national guidelines. Audits do happen, and the consequences of skipping documentation aren’t just paperwork—they might end up halting research or triggering hefty fines.
The core idea behind solid storage is respect. If you treat Piperidine-2-Methylamine with the care it deserves, accidents lessen, and the reliability of each result climbs. Real lab safety comes from daily habits: sealing bottles, labeling, cold storage, and paying attention to the rules. It’s a simple recipe, but it’s what keeps science moving forward—and everyone going home healthy at the end of the day.
Most of us expect a certain quality when buying ingredients, whether for a home recipe or a complex experiment. In the chemical world, purity isn’t a frill—it decides what you’ll get out of a process. Piperidine-2-Methylamine, a molecule used in labs and industries, shows this every time someone orders a bottle. Almost all suppliers offer this compound at 97% or higher purity, with some reaching above 99%. That little percentage might not seem like much, but it shapes outcomes. In pharmaceuticals and advanced materials, even a trace impurity can tangle up the results. A clean profile means reliability. Chemists want data they can trust, and product developers don’t want to troubleshoot unnecessary mysteries.
I remember my university days, waiting for a shipment, hoping everything inside matched what the label promised. A batch with less than stated purity often ended the lesson before it started. Piperidine-2-Methylamine, being both reactive and sensitive to contamination, calls for close quality controls. Leading producers back up purity claims using modern techniques like GC-MS and HPLC, so researchers and manufacturers know what’s inside before opening a container.
Not every project needs the same amount. For Piperidine-2-Methylamine, suppliers try to cover both small research needs and large-scale applications. Researchers usually look for bottles as small as 1 gram or 5 grams, letting them run tests without a stockpile. For those making bigger batches—think pilot plants or custom synthesis—bags, drums, or even intermediate bulk containers (IBCs) offer options running from 100 grams up to 25 kilograms or more.
A former colleague in industrial R&D told me how frustrated he’d get hunting for mid-sized quantities. The usual data sheets would rattle off the highest and lowest options, but place an order between those numbers and things got complicated. Good suppliers listen and stock 500 gram and 1 kilogram bottles, not just awkwardly large drums or tiny vials. This flexibility keeps projects moving and budgets intact.
How a chemical is packed does more than manage how much sits in storage. Piperidine-2-Methylamine, with its reactive amine functionality, can degrade in contact with air or incompatible plastics. Industry-standard glass or heavily lined HDPE containers cut risk and help maintain that high-purity number, even after repeated openings. Proper sealing, child-resistant caps, and clear hazard labeling all speak to practical experience: no one wants a surprise in the lab or on a factory floor.
For shipments across borders, every packaging choice has to meet rules from UN transport codes and national regulators. A surprise inspection revealing a leaking bottle or an unlabeled drum could bring a project to a screeching halt. Some suppliers offer tracked chain-of-custody for high-purity samples, making sure what you order is what you get, from order confirmation to the receiving dock.
The chemical market works best when buyers get clarity and consistency. Publishing batch-specific purity data and certificate of analysis, labeling every package size clearly, and offering tailored packaging helps both sides. Retailers and distributors looking for ways to stand out do well to add more mid-sized options and improve their customer support teams’ product knowledge.
Open communication between buyer and supplier about storage, purity needs, and handling goes a long way toward smooth, safe progress in the lab and on the production floor. For Piperidine-2-Methylamine, every percent and every gram counts.
| Names | |
| Preferred IUPAC name | 2-(Aminomethyl)piperidine |
| Other names |
2-Aminomethylpiperidine Piperidin-2-ylmethylamine 2-Piperidinemethanamine |
| Pronunciation | /paɪˈpɛr.ɪˌdiːn tuː ˌmɛθ.ɪl.əˈmiːn/ |
| Identifiers | |
| CAS Number | 16152-06-0 |
| 3D model (JSmol) | `JSmol.loadInline("data/model/cml,Piperidine-2-Methylamine")` |
| Beilstein Reference | 102229 |
| ChEBI | CHEBI:18714 |
| ChEMBL | CHEMBL3214838 |
| ChemSpider | 16736726 |
| DrugBank | DB08398 |
| ECHA InfoCard | 100.139.770 |
| EC Number | 202-490-2 |
| Gmelin Reference | 7674 |
| KEGG | C06182 |
| MeSH | D03.383.129.308.514.725 |
| PubChem CID | 122298 |
| RTECS number | TK3150000 |
| UNII | IQ3568NA2U |
| UN number | UN2735 |
| CompTox Dashboard (EPA) | DTXSID5033704 |
| Properties | |
| Chemical formula | C6H14N2 |
| Molar mass | 100.18 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | Amine-like |
| Density | 0.88 g/mL at 25 °C (lit.) |
| Solubility in water | Soluble in water |
| log P | -0.2 |
| Vapor pressure | 0.5 mmHg (at 25 °C) |
| Acidity (pKa) | 11.2 |
| Basicity (pKb) | 3.25 |
| Magnetic susceptibility (χ) | -6.17 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.482 |
| Viscosity | 1.12 cP (20°C) |
| Dipole moment | 2.11 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 253.6 J·mol⁻¹·K⁻¹ |
| Hazards | |
| GHS labelling | GHS02,GHS05,GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Harmful in contact with skin. Causes severe skin burns and eye damage. Harmful if inhaled. May cause respiratory irritation. |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| Flash point | 74 °C |
| Explosive limits | Lower: 1.7% ; Upper: 10.9% |
| Lethal dose or concentration | LD50 (oral, rat): 470 mg/kg |
| LD50 (median dose) | LD50 (median dose): 430 mg/kg (rat, oral) |
| NIOSH | SKC35700 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Piperidine-2-Methylamine: Not established |
| REL (Recommended) | 0.05 ppm |
| IDLH (Immediate danger) | IDLH: Not established |
| Related compounds | |
| Related compounds |
Piperidine 2-Methylpiperidine Piperidine-3-methylamine Piperidine-4-methylamine Piperidine-2-carboxylic acid N-Methylpiperidine 2-Aminopiperidine Morpholine Piperazine Pyrrolidine |