Piperidin-3-ol has roots going back to early 20th-century organic chemistry. Chemists originally searched for versatile building blocks that could bridge the gap between simple amines and more complex heterocycles. Over time, structural analogs of piperidine shaped the development of synthetic methods for pharmaceuticals and fine chemicals. By the late 1950s, research journals featured procedures for converting piperidine rings to various hydroxylated forms, including the coveted 3-position alcohol. Those early papers speak to a period of fast-paced progress. During the 1970s, as laboratory standards improved and analytic tools advanced, the isolation and purification of piperidin-3-ol became far simpler, encouraging chemists to further explore its potential in both academia and industry.
Today, piperidin-3-ol finds its way into research labs, specialty chemistry companies, and pharmaceutical synthetic routes. Its unique structure—a piperidine ring bearing a hydroxyl group at the third carbon—sets it apart from other secondary amines. The compound serves as an intermediate, facilitating access to more complicated molecules. Its uses include the synthesis of active pharmaceutical ingredients, agricultural chemicals, and even some specialty polymers. Bulk suppliers often provide this material in stable, recyclable packaging, with purities exceeding 98%, reassuring chemists about the reliability of their starting material.
At room temperature, piperidin-3-ol typically appears as a colorless to pale yellow liquid or low-melting solid, depending on its exact purity. Its molecular formula reads C5H11NO and the structure features both nitrogen and oxygen atoms, making it amphiphilic enough to dissolve in both water and many organic solvents. The presence of a secondary alcohol means the compound can participate in hydrogen bonding, and this plays a key role when designing reaction pathways that leverage its unique shape and reactivity. Its boiling point hovers around 205°C, and its pleasant, slightly sweet odor can mask the typical bitterness expected from simple amines, which is a small but practical bonus during lab work.
Manufacturers supplying piperidin-3-ol must adhere to detailed labeling requirements. Each shipment includes lot numbers, production dates, and documented origins for full traceability. Product labels highlight purity percentages, methods of purification, and analytic data such as NMR and GC-MS spectra. This detailed information enables researchers to quickly audit the quality and integrity of the compound before it enters a critical synthesis. Labels frequently list the melting and boiling points, storage conditions, and emergency information. Reputable suppliers routinely test for residual solvents and inhibitors to keep contamination below acceptable thresholds, reassuring anyone who ever questioned the purity of what arrived in their lab.
Most commercial routes for manufacturing piperidin-3-ol involve the partial reduction of pyridine-3-carboxylic acid derivatives, followed by hydrolysis and hydrogenation steps. Chemists can carry out this multi-stage synthesis under mild pressures using selective catalysts such as palladium on carbon, avoiding aggressive conditions that could degrade the ring. Hydrogenation setups inside modern reactors produce high yields, especially when optimized with sufficient agitation, precise temperature control, and constant monitoring by high-performance liquid chromatography. Laboratories working at smaller scale often adapt these published methods, modifying them to suit available reagents or to streamline purification, for example, by short-path distillation or crystallization from alcohols.
Once you have piperidin-3-ol, the door opens to a wide range of chemical transformations. The hydroxyl group, sitting at the third carbon, invites etherification, esterification, oxidation, and halogenation. For example, simple treatment with acid chlorides or anhydrides gives esters, while mild oxidation can convert the alcohol to a ketone. The nitrogen in the ring offers further functionalization, enabling the formation of N-alkyl or N-acyl derivatives. In medicinal chemistry, chemists often tweak both the hydroxy and amino groups to adjust solubility, bioavailability, or binding affinity. These modifications impact the compound’s behavior and help tune the pharmacological profiles of many final products.
Piperidin-3-ol sometimes goes by alternative designations to suit different markets. Synonyms in the literature include 3-Hydroxypiperidine, 3-Piperidinol, and beta-hydroxypiperidine. International suppliers may label it using these names, and regulatory agencies require all synonyms on safety data sheets. Tracing these names through the literature or chemical catalogs helps researchers uncover relevant data and cross-check specifications before making critical sourcing decisions.
Handling piperidin-3-ol involves more than just gloves and goggles. Reliable lab safety depends on knowing its hazards—moderate toxicity, potential irritation to skin and eyes, and a faint but persistent smell that some find unpleasant. Material Safety Data Sheets call for well-ventilated spaces, chemical-resistant clothing, and routine spill management plans. Fume hoods prevent inhalation, and secondary containment limits the spread of accidental releases. Some schools and companies even provide extra training on handling nitrogen-containing organics due to their unique toxicological risks. Fire safety remains a priority, even though the compound is not highly flammable. Emergency eyewashes and showers should always be accessible, since accidental splashes require prompt, thorough rinsing.
The value of piperidin-3-ol comes alive in the world of applied chemistry. Medicinal chemists rely on it as a scaffold for drug discovery, especially for central nervous system agents, antiviral compounds, and enzyme inhibitors. In agrochemical research, it shows promise for synthesizing active components that control pests or enhance crop resistance. Materials science benefits, too, as polymer researchers experiment with modifying piperidine rings to tune electrical or thermal properties. Its dual functionality—both amine and alcohol in the same ring—lets scientists tailor it further, one group at a time, to headline new product releases across pharmaceuticals, biotechnology, and advanced materials.
Each year, research groups publish new methods for improving the yield, selectivity, and scalability of piperidin-3-ol synthesis. Catalytic hydrogenation procedures continue to make headlines, with scientists reporting new ligands, catalysts, and reactor designs. Collaborations between academic and industrial labs speed up the evaluation of new derivatives, aiming to replace harsh reagents with safer, greener alternatives. Computational modeling grows in influence, letting teams predict the behavior of novel piperidine-based drugs before they ever enter the lab. Peer-reviewed journals and patent offices have seen a steady rise in publications and filings that include piperidin-3-ol scaffolds, suggesting that both fundamental science and commercial innovation feed off the versatility of this one molecule.
A responsible approach involves not only maximizing utility but also thoroughly investigating safety risks. Piperidin-3-ol demonstrates moderate acute toxicity in animal models, with irritation to mucous membranes, respiratory tracts, and skin. Chronic exposure remains underexamined, so caution dictates working with small amounts, careful storage, and detailed hazard communication. Regulatory reports cover the typical occupational exposure risks, from headaches and nausea to more severe effects if ingested or inhaled in large quantities. Toxicologists keep searching for safer analogs or additives that can minimize the impact on both users and the environment, advocating for transparent reporting and regular screening of exposed personnel. Until toxicity data catches up to usage rates, prudent handling and full compliance with safety protocols protect everyone involved.
Looking ahead, the outlook for piperidin-3-ol includes both incremental advances and the promise of major breakthroughs. Trends point toward greener, more efficient synthetic routes. Chemists invest in renewably sourced starting materials and aim to cut down on hazardous reagents. Regulatory agencies expect even more transparency on product documentation and lifecycle analysis, so chemical suppliers are putting serious resources into traceability and environmental impact studies. Medicinal chemists hope to unlock new therapeutic opportunities with analogs tailored for diseases that resist older drugs, adding further importance to this molecule’s role in research pipelines. By embracing responsible innovation and data-driven approaches, scientists keep pushing the boundaries of what piperidin-3-ol can bring to lab benches, production floors, and patient lives around the globe.
Piperidin-3-ol might have an awkward, forgettable name, but chemists know its real value. It’s a building block in drug chemistry and a backbone for research that pushes medicine forward faster than most folks realize. On its own, Piperidin-3-ol doesn’t treat any illness, yet researchers lean on it to design and improve all sorts of drugs—think antidepressants, painkillers, and antiviral agents.
Drug development keeps getting tougher as diseases evolve and old treatments lose their punch. Piperidin-3-ol helps turn tough chemistry problems into possibilities. Its structure makes it a cornerstone for synthesis—the art of making one kind of matter into something completely new. With its mix of an alcohol group and a piperidine ring, scientists link it to other molecules, shaping new compounds that sometimes hold the secret to treating stubborn infections or neurological conditions.
Plenty of modern pharmaceuticals owe their strength or safety to clever tweaks involving Piperidin-3-ol. For example, several drugs aimed at balancing brain chemistry use it as a starting point because it offers the right flexibility for further modification. I’ve seen lab teams grind through dozens of potential ingredients, but Piperidin-3-ol often gets pulled off the shelf for its reliability in adding both stability and new reaction sites.
Chemistry’s reach doesn’t stop at medicine. Agricultural researchers also put Piperidin-3-ol to work, building safer and more effective pesticides. Its base structure blends well with other chemical groups to fine-tune a compound’s behavior out in the field. This means crops face fewer threats while the environment absorbs less collateral damage from unnecessary chemicals.
Polymer science taps Piperidin-3-ol for its stability. Since it can join forces with other monomers, it helps produce plastics and coatings that hold up against tough conditions. As engineers hunt for new ways to make materials both sturdy and recyclable, compounds like this one carry more responsibility than they used to.
Every chemical in a laboratory brings responsibilities. Piperidin-3-ol gives chemists an edge, but it comes with rules—handling, storage, and disposal all demand strict attention. As with many lab chemicals, accidental exposure risks range from skin irritation to more serious reactions if inhaled or swallowed. Regulators and companies share a duty: enforce clear labeling, invest in employee training, and regularly check that safety procedures don’t slip out of date.
Sourcing also tugs at supply chains. Researchers want reliable, high-purity batches without unexpected contaminants. Bad suppliers or clogged trade routes can stall groundbreaking research. Companies with a reputation for quality and transparency win out since even a little impurity ruins months of work or, worse, causes harm when testing moves beyond the bench.
People hope for smarter drugs, safer crops, and better materials each year. Piperidin-3-ol sits quietly behind many of these advances. For every breakthrough announced on the news, a web of simple molecules like Piperidin-3-ol waited patiently for scientists to unlock their promise. Supporting the safe and ethical use of such chemicals isn’t just a technical matter—it’s the foundation for trust in what science brings next.
Piperidin-3-ol isn’t just another shelf chemical. After spending years moving between university research labs and pharma manufacturing lines, I’ve learned to respect the hazards certain chemicals carry, even those with mild-sounding names. This one, with its nitrogen ring and alcohol group, packs some quirks. You’ll find reports of it irritating eyes, skin, and lungs. Some people end up with headaches or dizziness if the ventilation isn’t dialed in. Chemical burns are real if spills get ignored. It’s not explosive, but accidents don’t need fire to do damage. So before a single cap gets twisted, planning matters.
Anyone who’s worked in a well-run lab can tell you stories of that one time a glove saved them. With piperidin-3-ol, I gear up with splash goggles tight on my face, snug nitrile gloves, and a crisp lab coat or chemical apron. Gloves get swapped right away at the first sign of a leak or tear. Chemical-resistant sleeves help with bulk handling. Closed shoes stay on the feet, not below the bench. I’m not interested in seeing what this stuff does to skin or eyes.
Few things bother me more than a coworker ignoring the fume hood. Piperidin-3-ol’s fumes might seem mild until a long day brings on a sore throat or foggy head. I never handle it outside the hood. Storage gets its own spot—away from acids, oxidizers, and open flames. Metal cabinets with proper ventilation and solid labeling keep the inventory honest. I double-check the lids and never leave materials open, even for a minute. Any old bottle with a damaged seal goes straight to chemical waste, not back on the shelf “for now.”
Even careful hands drop things. The difference is how you react. Small spills—grit, towels, and a dash of sodium bicarbonate neutralize the area fast. Bigger splashes mean evacuating the room, calling in trained response, and double-checking air quality before anyone returns. I’ve seen folks rush back before testing, and it’s not worth the risk. Skin contact means rinsing for a full fifteen minutes under running water and reporting to the onsite medic. I keep the eyewash station clear and never let clutter block my exit.
A written safety plan looks neat on the wall but falls flat if nobody cares. I show new hires the right way to suit up and quiz them hard before anyone opens a container. The group stays safer when even the over-confident chemists stick to the same playbook. Updates come with every change, and I encourage everyone to speak up about near-misses. The real lesson is to never let routine blind anyone to risk. In my experience, a team that shares stories about close calls stays sharper and friendlier.
Proper disposal keeps my mind at ease after a project wraps up. Piperidin-3-ol waste gets sealed in labeled containers and logged by date and volume. I avoid pouring anything down a sink, no matter what someone else claims is fine in low concentrations. Licensed vendors pick up regulated waste on a set schedule, and I keep receipts on file for inspections. No shortcuts here—the potential for harm never stays small, and tracking every step pays dividends if there are questions down the road.
Chemistry holds a special place in solving everyday problems, even if most people rarely think about molecules like piperidin-3-ol. This compound crops up when researchers and manufacturers explore new medicines or specialty chemicals. The name hints at its structure—a piperidine ring, which forms the backbone of countless pharmaceuticals. The buzzword here, “molecular formula,” draws the line between kitchen-table curiosity and the deep dives of professional labs. Knowing the precise arrangement and count of atoms in piperidin-3-ol matters far beyond trivia night. It anchors research, guides safety protocols, and connects the dots in drug development.
Piperidin-3-ol sounds like a mouthful, but breaking it down shows a clear picture. At its core, this molecule contains six carbon atoms, thirteen hydrogen atoms, one nitrogen atom, and one oxygen atom. Taken together, the molecular formula is C5H11NO. Each atom counts: those carbons create the ring structure typical of piperidine; hydrogen brings stability; nitrogen and oxygen offer the unique functionality that lets chemists attach new groups or build bigger structures.
I have seen first-hand how minor changes in molecular structure produce major effects. One less hydrogen or an extra oxygen can shift a compound from helpful to harmful or from bland to something vital. Researchers rely on precise molecular formulas to avoid mistakes that cost time, money, or even safety. In a pharmaceutical startup’s lab, a surprising result once traced back to a small error in understanding the formula—showing how easy it is to stumble without careful attention.
Accuracy keeps teams on track, especially with compounds that serve as building blocks. Piperidin-3-ol’s structure lets it act as a stepping stone for drugs aimed at neurological diseases. A simple swap at the “3-ol” position often makes drug candidates either promising or ineffective. Regulatory agencies, industry chemists, and academic teams all lean on the molecular formula to chart the safest, most efficient paths.
Strict documentation saves lives and money. Mistakes with molecular formulas feed bigger problems. A wrongly listed formula on a safety data sheet could spark incorrect handling or expose workers to risk. Labs with robust checking systems—incorporating regular peer review and cross-referencing—notice fewer incidents and get more reproducible results. Educational programs, too, can shape habits early. High school chemistry may seem simple, but teaching learners the value of accuracy in formulas lays a strong foundation. Clear visual aids, hands-on models, and digital analysis tools help cement correct practices.
Research keeps evolving. As questions get tougher, reliability becomes non-negotiable. Chemists who treat molecular formulas as non-negotiable facts land closer to breakthroughs. Piperidin-3-ol serves as a lesson in attention to detail: projects that start with solid molecular knowledge build faster, safer, and more meaningful results. The right formula keeps doors open for collaboration, regulation, and discovery. Whether in the classroom, the lab, or the boardroom, a simple number of carbons, hydrogens, nitrogens, and oxygens can drive success.
Piperidin-3-ol stands out for both its utility in chemical synthesis and its potential hazards. This compound, used in research and some pharmaceutical pathways, poses challenges if left to sit in the wrong corner of the lab or production site. People who handle chemicals long enough learn—sometimes the hard way—that nothing slows down work like an unexpected spill or a container failure. That’s where proper storage pays dividends.
I’ve known colleagues who assumed a cabinet would do for all chemicals. Piperidin-3-ol isn’t so forgiving. Exposure to air can trigger slow but unwanted changes in some organic compounds, including oxidation. This leads to reduced purity and could compromise safety during a synthesis. Moisture often brings its own set of problems, encouraging unwanted reactions and altering the intended use of the material.
Someone once kept a flask of a related compound on a shelf by a sunny window. Temperature swings, bright light, and invisible humidity ruined weeks of work. Ever since, I look for a cool, dry, and dark spot before putting away any chemical like Piperidin-3-ol. A basic flammable storage cabinet fits the bill, but a refrigerator made for chemicals offers tighter control over temperature, especially in warmer climates or older buildings.
Glass or high-quality plastic vessels with airtight seals block both air and moisture better than budget plastic bottles. It’s tempting to reuse old chemical containers, but leftover residue or unfamiliar material can lead to contamination and, in worst cases, unexpected reactions. Labels should always feature clear names and dates. It’s not about satisfying a regulation—it saves people from digging through paperwork during an emergency.
I can recall nights spent searching for proper container sizes only to find mismatched caps or cracked seals. Spending a few extra minutes checking each bottle and seal makes a long-term difference. It helps stop vapors from escaping, prevents spills, and keeps the work area much safer.
Authorities don’t set chemical storage standards just to create paperwork. They base their guidelines on incidents and scientific studies. In the U.S., the Occupational Safety and Health Administration (OSHA) and the Environmental Protection Agency (EPA) both offer direction, especially for workplaces. Europe has its own REACH regulations, often calling for similar practices. Ignoring these rules exposes organizations to hefty fines and, more importantly, significant risks to people and property.
In one incident I witnessed, an unmarked bottle containing a volatile amine ended up in a regular waste basket. Quick detection and a fast-thinking team prevented a fire. Emergency plans, including accessible eyewash stations and spill kits nearby, limit the impact if storage ever goes wrong. Staff briefings and regular inventory checks also cut down on accidental exposures.
While it might sound obvious, double-checking chemical inventories and cleaning out outdated materials close the loop on safe storage. This reduces the risk of forgotten bottles creating trouble months or years later. Handling Piperidin-3-ol with care isn’t just about following rules; it’s about looking out for colleagues and keeping research on track.
Piperidin-3-ol, a chemical compound with applications in pharmaceuticals and research, stirs up questions about access and regulation. Many folks in labs and in industry have probably run into roadblocks while trying to source chemicals with potential dual uses. It’s not just about scientific curiosity; the law and best practices steer most of the conversation.
Most reputable suppliers dealing with Piperidin-3-ol focus on business-to-business transactions. Individual buyers looking to pick it up for a personal project will likely hit a dead end. Trusted chemical suppliers—think Sigma-Aldrich, Alfa Aesar, TCI Chemicals—won’t ship to residential addresses. They expect clear proof that the order is coming from a legitimate business, university, or government lab. Buyers get vetted, licenses checked, end-use statements collected, and sometimes, additional paperwork falls into place if the chemical has sensitive status in a region.
Not every supplier keeps Piperidin-3-ol on their digital shelf. Of those that do, the minimum you can buy depends on the company’s policies and packaging. Labs usually spot quantities like 1-5 grams, while bigger outfits supplying in bulk start at 25 grams, 100 grams, or even kilograms. Minimum order quantities act as filters, screening out non-serious requests and improving inventory management. From my time in a research setting, I noticed we rarely got to pick and choose exact amounts, and over-ordering led to waste or regulatory headaches.
Pricing isn’t transparent for everyone. Suppliers may put a price tag online, but for controlled or restricted substances, a formal quote process happens behind the scenes. Expect Price On Request (POR) listings more than an easy “Add to Cart.” Add currency fluctuations, shipping rules, and hazmat fees, and the cost climbs fast. Following up directly with the supplier gets the most accurate answer about stock and price changes, especially for niche compounds like Piperidin-3-ol.
Access to chemicals with psychoactive or precursor potential sits under a microscope. Piperidin-3-ol isn’t exempt. Regulations vary: in the United States and Europe, sometimes it falls under voluntary reporting, and sometimes stricter rules apply. Anyone who has ever filled out DEA or precursor chemical forms knows they take time, and a missing signature means a shipment sits in limbo. A rush buy can lead to compliance trouble, so no company wants to risk its license on an unverified sale. The barriers can feel frustrating, but they exist for public health and safety.
For research and industry, building trust with established suppliers cuts through red tape. Developing relationships with vendors can open lines of communication, leading to easier collaboration, smoother paperwork, and early heads-up on regulatory shifts. For buyers, keeping documentation up-to-date and knowing the paperwork drill isn’t just bureaucracy—it’s a practical necessity. Firms interested in Piperidin-3-ol should regularly consult regulatory agencies for updates, train staff on compliance, and invest in secure handling systems to protect both people and business operations. Ethical sourcing, robust documentation, and informed negotiation with suppliers allow critical research and development to continue without unnecessary roadblocks.
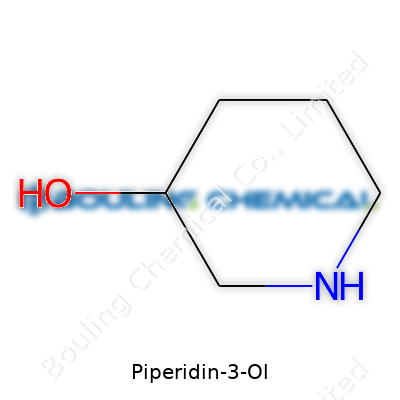
| Names | |
| Preferred IUPAC name | 3-Piperidinol |
| Other names |
3-Hydroxypiperidine Piperidin-3-ol Piperidine-3-ol 3-Piperidinol |
| Pronunciation | /paɪˈpɛrɪdɪn θri ɒl/ |
| Identifiers | |
| CAS Number | “62011-91-2” |
| 3D model (JSmol) | `3D model (JSmol)` string for **Piperidin-3-ol**: ``` CC1CCNC(C1)O ``` This is the SMILES string for Piperidin-3-ol, which can be used in JSmol to generate its 3D model. |
| Beilstein Reference | 1358730 |
| ChEBI | CHEBI:189295 |
| ChEMBL | CHEMBL16241 |
| ChemSpider | 177232 |
| DrugBank | DB08317 |
| ECHA InfoCard | ECHA InfoCard: 100_013_665 |
| EC Number | 200-537-7 |
| Gmelin Reference | 82917 |
| KEGG | C05962 |
| MeSH | D04722 |
| PubChem CID | 123517 |
| RTECS number | TK8400000 |
| UNII | 2BU7KH55VI |
| UN number | UN2810 |
| CompTox Dashboard (EPA) | DTXSID50865244 |
| Properties | |
| Chemical formula | C5H11NO |
| Molar mass | 101.16 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | amine-like |
| Density | 0.9 g/cm3 |
| Solubility in water | soluble |
| log P | 0.55 |
| Vapor pressure | 0.5 mmHg (25 °C) |
| Acidity (pKa) | 10.8 |
| Basicity (pKb) | 2.80 |
| Magnetic susceptibility (χ) | -72.8·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.474 |
| Viscosity | 90 mPa·s |
| Dipole moment | 2.33 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 274.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -146.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4276.6 kJ/mol |
| Hazards | |
| Main hazards | Causes skin irritation. Causes serious eye irritation. Harmful if swallowed. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | `OC1CCCNC1` |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | Precautionary statements for Piperidin-3-ol are: "P261, P280, P305+P351+P338, P337+P313" Let me know if you need the full precautionary phrases. |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 77.9 °C |
| Autoignition temperature | 270°C |
| Lethal dose or concentration | LD50 (oral, rat): 866 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 368 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 200-500 mg |
| Related compounds | |
| Related compounds |
Piperidine 3-Piperidone Piperidin-4-ol Nipecotic acid 2-Piperidone |