Long before researchers gave Piperazine-2-One a spotlight in today’s laboratories, chemists in the late 19th and early 20th centuries learned to blend cyclic urea derivatives, investigating the effects of ring closures and substitutions. Early work tried to harness these structures for their medicinal properties. As organic chemistry matured, especially in pharmaceutical development, this compound emerged as a recurring feature in the study of central nervous system agents, antihelminthics, and specialty polymers. The memory of thick glassware and musty chemical catalogs brings to mind how far instrumentation and purity have come, with modern synthesis far more precise than the cruder methods of the past.
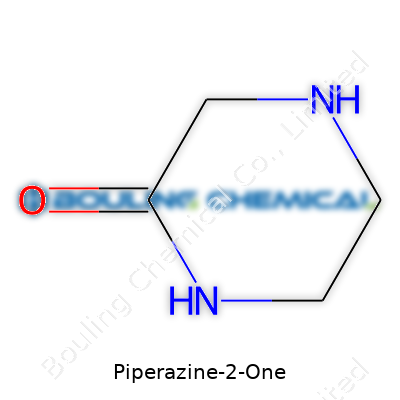
Piperazine-2-One stands out as a six-membered heterocycle containing two nitrogen atoms and a carbonyl group at the second position. Unlike the more straightforward piperazine, this derivative brings new reactivity due to its lactam ring. Most suppliers deliver it as a crystalline solid. A creamy white appearance, moderate melting point, and moderate solubility in polar solvents make it reasonably easy to work with, whether you’re in a pharmaceutical plant or an academic lab prepping for the next round of reactions. Commonly known under names such as 2-oxo-piperazine or sometimes piperazinone, depending on industry naming trends, this compound offers versatility for modifications on either nitrogen or adjacent carbons, attracting both medicinal and industrial chemists.
Most references put its melting point between 120 and 124°C—this range reflects the kinds of minor impurities you’ll see in all but the most rigorously purified samples. Its molecular formula, C4H8N2O, keeps things simple, but the presence of a carbonyl inside the ring gives the molecule a distinct reactivity. Limited water solubility but a higher affinity for solvents like ethanol and methanol hints at both challenges and benefits during synthesis and extraction. Its moderate stability under normal storage conditions means that long-term storage rarely brings surprises if containers remain dry and out of direct sunlight. The measurements—density close to 1.2 g/cm³ and a neutral pH when dissolved—fit easily into routine formulation work.
Commercial containers typically ship with purity ranging from 98% to >99.5%, a trace water content below 0.5%, and color indexes assessed visually. Labels list CAS number 7599-36-8, batch numbers, and sometimes impurity profiles when destined for pharmaceutical use. Standard practice advises sealed, opaque bottles with clear hazard warnings, such as eye and skin irritation risks, since accidental exposure to fine dust does not go unnoticed. Shelf life usually exceeds two years in tightly closed packaging, but even less than perfect storage rarely leads to significant degradation.
The straightforward method draws on cyclization of ethylenediamine with diesters or dicarboxylic acids, followed by mild dehydration. In a college lab years back, I watched the reaction as the mixture began to thicken and crystals started to form shortly after the cooling period. For a variant with higher purity, chemists often turn to catalytic hydrogenation of diketopiperazine derivatives. While large-scale producers use milder reagents with careful temperature control, the process works well on smaller scales. The reaction responds well to moderate heat and avoids noxious byproducts, making cleanup much simpler than some comparable ring-forming reactions.
Both nitrogens in the ring encourage acylation and alkylation. Medicinal researchers frequently attach small side chains at these positions or on the adjacent carbons to explore analogues with different biological activities. Switching up the carbonyl position or reducing it opens pathways to dozens of other heterocyclic compounds. One testament to its flexibility: many antipsychotic and anti-parasitic drug structures evolve from the core of piperazine-2-one by small tweaks. Reductions create piperazines, while oxidative conditions may yield ring-opened byproducts amenable to further reaction. Acid or base catalysis offers more control for selective derivatization, a handy trick for those wanting to make only one type of functional group modification without overhauling the whole system.
Beyond Piperazine-2-One and 2-oxo-piperazine, suppliers refer to this chemical as Diketopiperazine for related forms, though the two compounds aren’t always interchangeable. The pharmaceutical industry uses even more specific names based on substitutions and isomerism. Academic literature sometimes calls it Piperazinone or cyclic Glycylglycine. These subtle differences in naming sometimes lead to confusion, so double-checking CAS 7599-36-8 can save more than one chemist from an embarrassing experimental mix-up.
OSHA and local regulatory agencies recommend gloves and eye protection, partly because the powder floats easily and stings if it finds skin or mucous membranes. Dust extraction hoods prove invaluable in lab settings. Accidental inhalation or ingestion can cause dizziness and gastrointestinal discomfort. Often forgotten, clean storage spaces and routine checks cut down on contamination risk, especially when working with multi-step syntheses that amplify trace impurities. SDS (Safety Data Sheets) regularly highlight the need for dry storage above freezing and away from strong acids or oxidizers. Smart companies adopt procedures for quick spill cleanup and encourage keeping spill control materials within arm’s reach during large batch handling.
Pharmaceuticals top the usage list thanks to the central 2-one ring, a feature showing up in drugs targeting psychiatric disorders, as well as in antihelmintic medications that treat parasitic worm infestations. Beyond medicine, some researchers explore its structure in developing new polymeric materials, especially where controlled biodegradation matters. In analytical chemistry, piperazine-2-one derivatives offer reference standards for method validation and as substrates in enzyme-catalyzed reactions. Its low toxicity—at least compared to more reactive ring systems—lets it serve as a building block for numerous industrial and academic projects with minimal environmental management headaches.
At the frontier of research, most focus centers on tailoring the ring’s substitution to tweak biological properties—chasing selective inhibition of certain pathways or improving oral bioavailability. Structure-activity relationship studies repeatedly find that new side chains or altered ring sizes on this core yield better candidates for treating neurological disorders and infections. Polymer chemists keep investigating how these rings break down under physiological conditions, hoping to discover the next generation of drug delivery materials or bio-friendly plastics. Academic research keeps turning out graduate theses on ways to make synthesis even more efficient, reducing solvent waste and energy input.
Most safety data show only mild toxicity in animal models, with effects requiring relatively high doses. Acute exposure leads to minor gastrointestinal and neurobehavioral symptoms, which usually resolve once access stops. Chronic testing rarely triggers serious organ effects. Despite reassuring findings, regulatory bodies call for further toxicology studies, especially as more derivatives enter pharmaceutical development. Lab animals expose the limits of current knowledge, particularly with repeated or long-term dosing, which pushes researchers to keep refining dosing guidelines and preclinical screening methods.
Ongoing refinement in synthesis—especially adoption of greener chemistry approaches—points toward more sustainable industrial application. In medicine, growing recognition of the ring’s potential for custom tailoring matches the rise of personalized treatments for neurological and infectious diseases. Advances in material science could open new uses, as biodegradable functionalized rings might solve persistent issues with polymer breakdown and trace residue in consumer products. With regulators and environmental watchdogs pushing for safer chemicals, sustainable and efficiently synthesized piperazine-2-one derivatives may soon feature more heavily in both pharmaceuticals and everyday products. As research pushes boundaries, more laboratories and manufacturers will look to this structure for breakthroughs in both human health and material engineering.
Piperazine-2-one pops up all over chemistry discussions, but it rarely gets much attention from folks outside labs and pharmaceutical businesses. This small molecule stands out for a reason. Its structure gives it a backbone used by chemists looking to create new medicines. Having worked in a lab where turning powders into pills was just another day, I can remember how often piperazine-2-one ended up in the plans for drug synthesis.
Let’s talk real-world use. Many drugs sitting on pharmacy shelves started life as piperazine-2-one, or rather—the molecule offered a starting point. It serves as a “building block” in drug design, making it possible to snap other chemical groups onto it. This approach can lead to antibiotics, antipsychotics, cancer treatments, and more. Plenty of hard-working medicinal chemists rely on it for one simple reason—it’s flexible in how it joins up with other chemicals.
If you’ve ever heard about drugs called piperazines, this one is at the core. And it’s not just about coming up with new medicines. Some researchers use it as a jumping-off spot to improve old medications, reduce side effects, or change how quickly a drug breaks down in the body. Drug patents hinge on small chemical changes, and piperazine-2-one plays a role in giving new life to these compounds.
Piperazine-2-one sneaks into other industries, too. Lab folk sometimes use it to make things like agrochemicals, which help protect crops. It’s also used as a raw ingredient for dyes or specialty coatings. In those cases, it’s the chemical backbone that makes it valuable, since it’s stable and easy to transform.
This chemical might not have a headline-grabbing reputation, but behind the scenes it does heavy lifting. I remember colleagues poring over patents and published studies, tracing the path from simple piperazine-2-one to cutting-edge cancer therapies. Sometimes it shows up in academic research as a target—scientists want to learn more about how changing the rings or attachments around it alters activity.
It doesn’t take long digging through public chemical inventories to see just how often this molecule is chosen for research. For example, the ChEMBL database lists thousands of compounds built from the same ring system. In labs and tech companies, teams often run screening tests on hundreds of molecules based on it, searching for the next breakthrough treatment or tool for biology studies.
With all this interest, there’s a downside—overusing one chemical scaffold in drug design can limit the types of medicines that make it to market. If every drug company picks the same skeleton, we end up with many drugs acting in similar ways. A narrow focus could slow progress on treatments for diseases with no easy fix, like certain brain disorders or tough bacterial infections.
One fix comes from shifting the focus outward. Chemists who try new twists on piperazine-2-one—or decide to explore different molecular backbones—open up new directions for invention. Business leaders funding pharmaceutical research can back projects that don’t just follow industry trends. Taking a few well-calculated risks in research could pay off for patients, who ultimately care about new treatment options more than chemistry popularity contests.
Step into any pharmacy, you’ll find medications shaped by the tiniest molecules. Every so often, you run into something like Piperazine-2-One. Maybe you spot the name on a chemical safety sheet. Maybe a research article slips it in, talking about new drugs or industrial chemicals. We catch these names and rarely stop to wonder about the little details, like what the formula for these things really means and how it connects to much bigger realities.
Piperazine-2-One, chemically known as 2-piperazinone, builds its identity on a six-membered ring: four carbon atoms and two nitrogen atoms. There's something familiar about this skeleton. It’s almost like an art student blending just a few primary colors to form something entirely new each time. Here, you get a simple molecular formula: C4H8N2O. Behind those few letters and numbers is a story about how things stick together, bend, and react. Not glamorous, yet it shapes how this molecule interacts — with your body, or as an industrial tool.
Chemistry books throw formulas around like confetti. That’s fine until you realize how a single oxygen or a missing hydrogen changes everything. Piperazine-2-One with its C4H8N2O formula represents more than ink on a page. One extra atom could turn a harmless compound into a toxin. Or do the opposite. It’s personal, too. I remember visiting a friend who worked in drug discovery. Rows and rows of bottles lined the lab benches, each label a quiet reminder that formulas decide if a drug helps or harms. Missing a single oxygen, and your intended brain-calming medication could do nothing, or suddenly trigger a side effect nobody expects.
This compound often pops up as a building block. You find tweaks and modifications of the core formula in everything from anti-worm medicines to potential treatments for the central nervous system. My high school chemistry teacher told us, “Every time a scientist sees a piperazine ring, they see somewhere new to attach, add, or subtract.” These subtle changes carve out medicines that work on very specific pathways, sort of like fine-tuning a lock so only one key fits.
When companies request production at scale, knowing the formula keeps costs predictable and outcomes reliable. One of the first steps in making a drug goes like this: check the formula, confirm the arrangement, compare it against known allergens, and calculate what sort of by-products could result. Teams spend hours peering at these chains and rings. In my own undergraduate project, we puzzled for weeks over whether a tiny rearrangement would impact the stability of a related molecule. That lesson sticks with me — small molecular formulas change the game.
If you ever find yourself frustrated by science updates or legal labels, take another look at these formulas. They’re the handshake between discovery and everyday safety. Piperazine-2-One’s formula, sitting there as C4H8N2O, invites chemists to experiment safely, helps manufacturers scale up production without misfires, and someday might anchor the next big medical breakthrough—all because every letter and number counts. Getting them right saves lives, time, and money. Accuracy at this level protects trust between science and society.
Piperazine-2-one pops up often in conversations across labs and manufacturing floors. Pretty much every chemist will recognize the molecule for its value in pharmaceutical projects and crop protection chemicals. It delivers versatility most chemistries aspire to. Demand only increased as drug research picked up during the last decade. You’ll find it used as a building block for several drugs, making supplies crucial for seamless R&D, not just in headquarters but even in independent university labs.
Years back, I tried to order a batch of it myself for a medium-sized development run. At the time, it felt hit-or-miss. Key suppliers held small lots, and bigger orders meant several weeks, upfront payment, or jumping hoops through customs. No researcher loves that sort of uncertainty. Delays push costs up and stall work. The pain point echoed for other folks too. I heard the same story from a friend in generics: sourcing big enough lots to keep pilot runs—never mind full scale—on track gets stressful fast.
Global suppliers out of India and China tend to dominate the conversation. Here in North America, you’ll rarely find a shelf stock past a few kilograms. Local chemical houses often call around and end up sourcing through one of five or six big overseas producers. There’s nothing shady in this, but freight rates shift all the time, and new trade rules sometimes throw a wrench into shipments.
Some years, anti-dumping duties or sudden inspections push everyone to hold off or pay more. I saw prices double within a few months last year after just a couple of customs hiccups. This puts a strain on small and midsize buyers. Big pharma can forecast; smaller players scramble to lock in orders or risk shelf gaps.
Everybody wants to talk about volume, but quality doesn’t always keep up. I’ve flagged this with import orders: changes in color, water content variation, or trace metals sneaking in. Buyers should never assume consistency from lot to lot or supplier to supplier. The more intermediaries, the fuzzier the trail gets. In my own work, I found local analysts catching “off” results even with goods labeled from reputable houses, forcing retesting and slowing the benchwork.
For those concerned with scale, these missteps matter. If a kilo fails spec, nobody’s running a hundred. Even repackers sometimes can’t guarantee the same grade, and documentation grows thinner as the supply chain grows longer. Sourcing directly from original manufacturers—ideally after seeing their lab reports—spares a lot of downstream headaches.
If reliable, bulk Piperazine-2-one supply matters for your operation, relationships count. My best results came from working directly with suppliers who stood behind their product, sometimes even offering samples for batch validation. Cold calls to new traders risk confusion and wasted time. There’s real benefit in sample testing first, and then building a track record with a supplier.
Another concrete move: ask about contracts or forward agreements when buying larger volumes, especially if your schedule is tight. Payment terms, warehousing, and lead times often get more flexible once a purchase history develops. Some buyers in biotech and pharma now join purchasing consortiums, pooling orders to boost leverage and secure better deals on bulk. This spreads risk, and most of all, keeps labs and plants moving.
I've seen enough lab benches crowded with questionable containers to know that chemical storage isn't just about keeping things neat. Chemicals like Piperazine-2-One rely on proper storage to keep their stability and usefulness. A simple mistake, like leaving a cap loose or tucking a bottle away by a window, can turn a helpful substance into a headache.
Piperazine-2-One doesn't throw unexpected tantrums if treated right, but it can break down or lose effectiveness under certain conditions. Most lab-grade powders and solids, including Piperazine-2-One, get along best in a dry spot. Damp air encourages clumping and possible reactions you want to avoid. I always pick a tightly sealed container—glass with a solid screw cap beats flimsy snap-tops every time—so moisture stays out and spills become rare.
Direct sunlight never did chemicals any favors, either. A shelf or cabinet away from windows, heat sources, and bright lights works better. Ultraviolet rays can slowly change a compound or speed up decomposition. Every lab I’ve enjoyed working in had cabinets labeled for dark storage, sometimes with amber bottles offering an extra layer of protection from stray light. It’s a small investment that prevents a lot of waste.
Room temperature often seems “good enough,” but not every room matches the recommended 20-25°C sweet spot. A day when air conditioning fails can spike the temperature. Fluctuations like this matter because Piperazine-2-One holds steady at moderate temperatures but doesn’t appreciate freezing or being left near radiators. I’ve seen labels fade off and even containers split when chemicals get too hot or cold too quickly, and it’s never a fun cleanup.
It’s a smart move to check the SDS (Safety Data Sheet) for exact temperature guidance, but as a rule, if you’d keep chocolate or medicine in that cupboard, it probably works for Piperazine-2-One, too. Some scientists go further and use temperature monitors inside cabinets, especially in climates with dramatic swings. The cost is low compared to tossing out spoiled material or risking faulty results in research.
Storing buns next to onions ruins both, and the same goes for chemicals. Stashing Piperazine-2-One far from acids, strong bases, and oxidizers reduces risk. Once, a mix-up in storage led to a faint smell in our chem closet and a lost afternoon cleaning shelves. Clearly marked shelves and no crowding solve most of those issues.
Inventory sometimes gets overlooked, but it prevents old material from piling up. Dates on every container make life easy. I like using a marker right on the label so anyone can see when something arrived. It’s a small step that means less waste and more confidence down the line if someone questions a batch.
Clear labeling saves more time and headaches than any other single habit. Every jar or bottle of Piperazine-2-One in my workspace has a chemical name, not just a shorthand or code. Add hazard notes, any unusual precautions, and you’ve done yourself and your team a favor.
Anyone storing Piperazine-2-One should check up on containers regularly. A quick shake for caked powder or a glance at the shelf for spills takes just a moment but prevents bigger problems later. Safety in the lab isn’t just a rule, it’s a habit built through these routine choices.
Walk into any chemical storage space on a campus, and you’ll find bottles labeled with names that don’t roll off the tongue. Piperazine-2-One is one of those. It pops up in pharmaceuticals, odd bits of organic synthesis, and, from time to time, in research projects with scientists in goggles. But the real question that matters isn’t whether it’s hard to spell — it’s how worried someone should be if their hands, lungs, or workspace come into contact with this stuff.
Facts cut through confusion. According to the GHS classification (that’s the system regulators around the world rely on), Piperazine-2-One doesn’t wear a big red danger label like some of its close cousins. But just because nobody’s storing it in a locked cabinet marked “Hazard: Extreme!” doesn’t mean nobody should show respect. Breathing in the powder, getting it on your skin, or splashing it into your eyes should never be part of anyone’s day in the lab.
One study I found from my college days showed mild skin or eye irritation in lab mice exposed to similar compounds. Humans can sometimes react more sharply, though, especially if someone already has sensitive skin. The dust can tickle throats, and anyone who’s ever forgotten to wear a dust mask in a chemistry prep room knows it only takes a split-second for a mild cough to land you in an office with a stern-faced supervisor. Gloves and glasses don’t cost much, and they save weeks of regret.
The trickiest thing with chemicals like Piperazine-2-One is the way small risks add up over time. In theory, the main threat looks like irritation — not instant poisoning — but labs often use these compounds in processes that stir up dust or vapor without good ventilation. Poor airflow is a recipe for headaches, sore eyes, and short tempers. Once, I saw a sizable spill on a bench. The person who handled it without gloves washed her hands after, but her skin was red for the rest of the afternoon.
As for eating or swallowing any of this—nobody in their right mind should. The Material Safety Data Sheet reads like a nagging parent on this one: rinse your eyes for a long time, call poison control, don’t minimize the risk just because the symptoms don’t show up instantly.
No flashy solutions work better than basic good sense. Storing Piperazine-2-One in a cool, dry cupboard, away from kids and snacks, helps. Always check the gloves for holes before grabbing a bottle, and never skip the goggles. Fans or hoods for ventilation aren’t just décor—they block clouds of dust from sneaking into your lungs. Training helps keep the know-it-all accidents at bay. Students and staff deserve more than a quick run-through on safety, because overconfidence cuts corners.
Disposal matters, too. Dumping leftovers down a regular sink lines up trouble for water systems. Every university and lab I’ve worked in has strict protocols for collecting even the blandest chemical waste, and I’ve seen firsthand how much hassle gets avoided by following those rules.
Piperazine-2-One doesn’t belong on a list of notorious lab hazards. Most problems start when people take shortcuts or skip protection, not because the compound itself leaps up to cause harm. Education works better than panic when handling chemistry that sits somewhere between boring and potentially irritating. With a little care, Piperazine-2-One remains just another bottle gathering dust, not a headline story in the campus safety report.
| Names | |
| Preferred IUPAC name | 1,4-Diazinan-2-one |
| Pronunciation | /paɪˌpɛrəˈziːn tuː oʊn/ |
| Identifiers | |
| CAS Number | 1994-92-1 |
| 3D model (JSmol) | `3D model (JSmol)` string for Piperazine-2-One: ``` C1CNCC(=O)N1 ``` |
| Beilstein Reference | 120924 |
| ChEBI | CHEBI:5179 |
| ChEMBL | CHEMBL50452 |
| ChemSpider | 24799011 |
| DrugBank | DB00542 |
| ECHA InfoCard | ECHA InfoCard: 100.007.703 |
| EC Number | 3.5.2.11 |
| Gmelin Reference | 107063 |
| KEGG | C13947 |
| MeSH | D010871 |
| PubChem CID | 13368 |
| RTECS number | RR0350000 |
| UNII | YH2688DA5X |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C4H8N2O |
| Molar mass | 86.118 |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.17 g/cm3 |
| Solubility in water | Soluble in water |
| log P | -1.39 |
| Vapor pressure | 0.000158 mmHg at 25°C |
| Acidity (pKa) | 6.27 |
| Basicity (pKb) | 7.38 |
| Magnetic susceptibility (χ) | -77.0e-6 cm³/mol |
| Refractive index (nD) | 1.558 |
| Viscosity | 200 cP |
| Dipole moment | 3.54 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 245.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -238.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2369 kJ/mol |
| Pharmacology | |
| ATC code | N05CM19 |
| Hazards | |
| Main hazards | May cause respiratory irritation. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H312 + H332: Harmful if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | Precautionary statements: P261, P305+P351+P338, P337+P313 |
| Flash point | 103°C |
| Autoignition temperature | 340 °C |
| Lethal dose or concentration | LD50 oral rat 4750 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 4750 mg/kg |
| NIOSH | Not Listed |
| PEL (Permissible) | Not established |
| REL (Recommended) | 100 mg |