Chemists discovered Piperazin-2-one in the early part of the 20th century during a wave of exploration into nitrogen-containing heterocycles. As synthetic organic chemistry took off, laboratories in Europe and the United States refined early methods for its creation, mapping out its core structure through systematic experiments. The molecule’s utility quickly stood out; not many compounds from that era found as meaningful a foothold across so many industries as this one. Early patents focused on its use as an intermediate for dyes and medications. Academic and industrial teams alike studied its structure, recording their findings in journals now considered classics in chemical literature. The structure enabled researchers to modify the compound and generate a suite of useful derivatives. Building on foundational organic chemistry knowledge, Piperazin-2-one became a scaffold for drug discovery, influencing research projects even today.
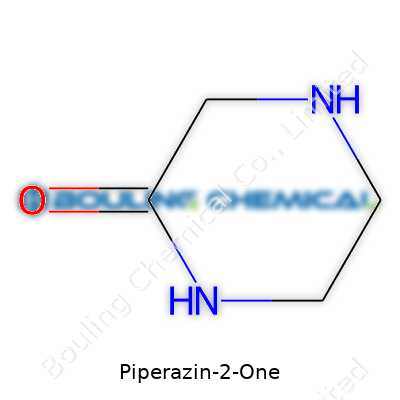
Piperazin-2-one occupies a unique spot in chemical libraries and research labs. The molecule combines a six-membered heterocycle containing two nitrogen atoms with a single keto functional group. Both medical researchers and manufacturers rely on this compound and its derivatives for their ability to serve as intermediates. Production typically involves a white to off-white crystalline powder, supplied by chemical companies in varying purities, but research-grade quality—above 98%—remains the standard for pharmaceutical work. Designers of bioactive molecules often use Piperazin-2-one as a core building block, modifying side chains to generate diverse pharmacological effects. Its straightforward chemistry allows both bulk production for industrial customers and custom synthesis for cutting-edge drug discovery labs.
Some properties make Piperazin-2-one particularly attractive for handling and processing. At room temperature, it presents as a stable, solid substance. Melting points usually span 140-150°C, an indicator of its suitability for a variety of chemical operations that require heat. Water solubility can be moderate, though solubility in polar organic solvents broadens its practical use. Its tautomeric nature offers room for chemical creativity, giving synthetic chemists a handle for further modification at both the nitrogen atoms and carbon positions. Ultraviolet absorption and infrared spectra lay out distinct signatures for contaminant analysis and quality control. Chemical stability extends shelf life in storage—key for research settings, where reagents often sit for months before use.
Reputable suppliers provide detailed technical documentation with batch deliveries. Specifications typically spell out molecular formula (C4H8N2O), molar mass near 100.12 g/mol, appearance, melting range, and any trace impurities. Purity is usually confirmed by HPLC and NMR, a must for medicinal research to avoid confounding results. Labels include storage instructions, hazard pictograms, and a lot number that ties the product to analytical reports and traceability records—elements governing both laboratory safety and compliance with local chemical control regulations. Chemical identity aligns with accepted IUPAC nomenclature, and packaging usually includes tamper-evident sealing, both for safety and preservation. The labeling standards meet regulatory requirements for transport, especially for export shipments.
Synthetic access to Piperazin-2-one developed through decades of study. The process often relies on cyclization reactions, where ethylenediamine derivatives react with suitable carbonyl sources under controlled conditions. Early methods made use of chloroacetyl chloride, but safety and environmental pressures led to greener alternatives such as utilizing safer acyl donors and milder bases in aqueous or high-boiling organic media. Reaction times and temperatures play a big role in yield and purity. Post-reaction, standard work-up steps—neutralization, extraction, crystallization—afford a clean product. Scientists in scale-up roles aim to reduce byproducts, limit exposure to hazardous reagents, and streamline purification. Process optimization draws on decades of cumulative know-how, including use of modern catalysts and continuous flow reactors for larger operations.
The piperazine core leads to vivid chemical landscapes. Chemists can perform alkylation or acylation at the nitrogen atoms to develop a wealth of N-substituted derivatives, many with proven activity as pharmaceuticals or agrochemicals. The carbonyl center provides an anchor for further reactions—reduction gives N-hydroxy derivatives, while condensation can attach more complex heterocycles or aromatic groups. Cross-coupling techniques using established palladium and copper catalysts open the door for structural diversity, helping researchers tailor properties for solubility, bioavailability, or receptor selectivity. The molecule’s compatibility with both acidic and mildly basic conditions supports flexible synthetic pathways. This flexibility explains Piperazin-2-one’s popularity in medicinal chemistry, as researchers can fine-tune molecular properties at multiple positions to probe structure-activity relationships.
Researchers and suppliers recognize Piperazin-2-one under several aliases. Common synonym lists include 2-Piperazinone, 2-Oxopiperazine, and hexahydropyrazin-2-one. Literature citations and substance registries cover these variants alongside CAS numbers, helping researchers avoid confusion when scouring patents or previous studies. Some pharmaceutical intermediates and specialty chemicals markets use trade names reflecting compound modifications; buyers should always confirm structure and specification before ordering for synthesis. Universal recognition and traceable nomenclature improve scientific communication and prevent costly mistakes, particularly in international projects with partners spread across continents.
Responsible handling stands at the core of lab and production safety. Piperazin-2-one, though less acutely toxic than high-risk chemicals, still requires gloves, goggles, and proper ventilation. Dust inhalation and skin contact should be avoided, with workbench setups built to control possible spills or airborne particulates. Storage guidelines advise keeping the product away from heat and moisture, as decomposition could yield volatile byproducts. Material safety data sheets detail hazard ratings and recommended first-aid actions, aligning with both OSHA and international chemical safety codes. Disposal policies steer spent materials toward certified chemical waste programs, keeping environmental contamination in check. Employees handling scale-up operations undergo training in emergency measures and safe container management, supporting both workplace safety and regulatory compliance.
Drug discovery and development strongly benefit from Piperazin-2-one. The scaffold features prominently in antipsychotic, antihistamine, and anticancer candidates, helping researchers build molecules that reach and bind specific biological targets. Pharmaceutical catalogs include dozens of derivatives currently under clinical evaluation for neurological and infectious diseases. Outside health care, Piperazin-2-one shows up in specialty resins, agrochemicals, and even materials science. Smaller applications span dye intermediates and corrosion inhibitors. Research teams find the molecule indispensable as a reference or starting material, with many leading journals tracking advances built on this core ring system. As synthetic tools improve, new fields—such as bioconjugation and polymer chemistry—have begun importing Piperazin-2-one derivatives for further study.
Momentum around Piperazin-2-one continues. Medicinal chemists probe new analogues by attaching designer fragments, hoping to overcome resistance or side effect limitations of existing drugs. Pharmaceutical companies partner with academic groups to review compound libraries, in search of profiles that fit target product criteria like potency and pharmacokinetics. Green chemistry initiatives have prompted exploration of solventless or water-based cyclization pathways to lessen the environmental footprint of large-scale production. In analytical chemistry, researchers use modern spectrometric and chromatographic methods to quantify trace levels of Piperazin-2-one in complex mixtures. Recent years also brought focus to computational modeling—researchers use predictive software to screen virtual piperazinone structures before embarking on time-consuming laboratory synthesis. This growing integration of digital and laboratory workflows signals a shift in how the industry approaches molecule design and process optimization.
Toxicology studies set out to protect both workers and end-users, so teams run a full suite of assays. Raw Piperazin-2-one rarely shows strong toxicity in single-dose exposures, but repeated or high-concentration contact affects organ function in animal models. Chronic exposure studies and genetic toxicity tests provide data for safety thresholds. Environmental fate is another active area; breakdown products under sunlight and in soil must not persist or build up in food chains. Regulatory filings now demand evidence for both acute and chronic exposures. Contract research organizations perform metabolism studies, mapping out how the liver and other organs process the molecule and its derivatives. These findings help scientists design safer analogues and support regulatory compliance in drug and material applications.
Next steps for Piperazin-2-one look promising. Banks of chemical libraries continue to treat this core as a platform for new therapies; AI-guided drug design, now entering industrial pipelines, leans on such known but under-explored scaffolds. Efforts to produce better-tolerated medication run up against resistance and side effects, so broadening the application of Piperazin-2-one derivatives remains a top research goal. Advances in synthesis—cheaper, cleaner, and applicable to large scales—open the door for materials science and manufacturing outside traditional pharmaceutical markets. Environmental regulations have also pushed chemists to build versions with lower persistence, and safer pathways for processing. As regulatory, social, and technical needs keep shifting, having a robust, flexible molecule like Piperazin-2-one in the toolkit allows chemists to keep pace with both challenges and opportunities. The legacy of this compound links early 20th-century curiosity with the next generation of chemical, medical, and environmental breakthroughs.
Piperazin-2-One doesn’t show up in daily headlines, but its story runs through biology labs and the pharmaceutical industry. This compound, tucked behind its tongue-twister name, shows up in research aimed at solving problems most people never worry about until they need a solution. My first encounter with Piperazin-2-One happened in a college organic chemistry class, where molecules like this got sketched on chalkboards and talked about as “core building blocks.” At the time, it didn’t seem like much, but working on drug development projects later on, its importance grew much clearer.
Drug makers look for molecules that can serve as a backbone for new treatments. Piperazin-2-One often fills that role. Sometimes, scientists tweak its structure just a bit to build new antibiotics or antiviral agents. A 2020 review in the Journal of Medicinal Chemistry details how Piperazin-2-One derivatives show up in compounds that fight bacteria which resist older drugs. That’s not just interesting news for researchers. Resistant infections cost families hard-earned money, bring extra hospital stays, and threaten lives. Every new tool helps.
Antipsychotic medicines and some antidepressants use Piperazin-2-One too. These aren’t headlines that get people talking the way blockbuster cancer drugs might, but they matter for people struggling with mental health. By giving chemists a foundation with flexible chemistry, Piperazin-2-One allows for small changes that lead to big effects in the body. The right alteration—just a swapped atom or two—means a medicine can get absorbed better, break down slower, or target brain receptors in new ways.
Not every use ends in a pill bottle. Some scientists use Piperazin-2-One in the quest to understand enzymes and proteins. Imagine trying to make sense of a thousand-piece puzzle without knowing where any of the edge pieces go. Molecules like Piperazin-2-One offer a way to test how enzymes work and where chemical reactions stall or succeed. Growing up, I watched relatives struggle with illnesses that no one could explain fully. It drove home why research matters, even if the details get technical. Using Piperazin-2-One for experiments sheds light on diseases that don’t have straightforward answers.
Manufacturing and handling Piperazin-2-One calls for safety. It’s a chemical, not a household remedy, and exposure outside professional labs can cause problems. Regulatory bodies set standards for how much can be safely handled and in which situations. Focusing on proper training means fewer accidents and better lab results. Researchers who care about their teams—and about future medicines—support funding for safety programs and transparent waste management. Public trust in science grows with this kind of responsibility.
Piperazin-2-One highlights how progress doesn’t always come from headline-grabbing discoveries. Sometimes, quiet compounds give researchers the pieces needed for better medicines, quicker diagnoses, or safer labs. Sharing these stories with friends and family, I’ve noticed that people appreciate the connection: the next time they pick up a prescription, part of the solution might start in a small lab flask with a molecule like Piperazin-2-One.
Piperazin-2-one stands out in labs and manufacturing spaces because of its structure: a six-membered ring with two nitrogen atoms and a ketone group on the second carbon. This foundation makes it part of not just pharmaceutical discussions, but also materials science. Folks working on new drugs or additives come across it pretty quickly, due to how its physical and chemical fingerprint sets the tone for what scientists and engineers can do with it.
Solid at room temperature, piperazin-2-one gives off a faint chemical odor reminiscent of amides. Its melting point lands near 145°C, holding up against the sort of heat that breaks down less-stable compounds. Solubility catches the eye for chemists, too. It dissolves in water and polar solvents. This matters for households using products containing derivatives, and researchers running purification steps in drug development. Its white or off-white appearance signals cleanliness in lab settings, letting users spot contamination or degradation without specialized tools.
Anyone handling it notices a crystalline texture, which affects both storage and handling. Less clumping means fewer headaches for folks using it in manufacturing setups or blending processes. Density sits at about 1.32 g/cm3, which means it behaves solidly during weighing and mixing. These characteristics ensure consistent measurements whether preparing analytical samples or scaling up for factory runs.
With a molecular formula of C4H8N2O, piperazin-2-one contains enough reactive sites to catch the attention of anyone designing pharmaceuticals or specialty materials. The carbonyl group, in particular, draws nucleophiles—making this molecule a useful starting point for further functionalization. It resists oxidation under regular storage conditions, but harsh oxidizers turn it into other nitrogenous fragments, so folks storing it tend to watch for incompatible chemicals.
This compound’s moderate basicity, thanks to the piperazine ring, opens doors for salt formation—an important step when preparing medications that must dissolve at predictable rates in the body. Thanks to these properties, pharmaceutical chemists build whole classes of drugs with it as a core.
Stability presents few problems in a well-sealed container, away from light and extreme heat. I’ve worked in shared lab environments where everyone appreciated predictability: no sudden degradation, few unpleasant surprises. That predictability means less risk for users, as hazardous decomposition products rarely show up—unlike some more finicky chemicals.
Anyone who’s tried to create new treatments for conditions like epilepsy or schizophrenia has probably worked with piperazin-2-one derivatives. Several approved medications trace their roots to this core structure. Its versatility keeps it relevant even as research priorities shift.
Despite its reliability, a few hurdles stand between lab and real-life applications. Handling protocols exist for a reason—its derivatives may produce unwanted side effects if not carefully designed and tested. Safety data highlights limited acute toxicity, but long-term exposure, especially in poorly ventilated manufacturing settings, needs attention. I’ve seen older factories struggle to keep dust and vapors below exposure limits. Routine monitoring and worker training make a difference, often backed by updated engineering controls that focus not just on compliance, but on real safety.
Improving both safety and sustainability around piperazin-2-one starts with education. New production techniques aim to minimize waste, solvents, and byproducts. I’ve seen young chemists passionate about green chemistry push for these upgrades in university labs and startup spaces alike. Transparent data sharing—such as letting researchers access real-time hazard and property data—fosters safe innovation without unnecessary risk.
In the end, a compound’s success in the real world depends as much on practical wisdom as it does on formal scientific traits. Piperazin-2-one’s properties make it valuable, but it’s the choices people make about its use and stewardship that shape its story in pharmaceuticals, industry, and beyond.
Most people outside chemistry labs probably haven’t heard of Piperazin-2-One. This compound pops up in a lot of pharmaceutical research. Sometimes, it even finds its way into drug manufacturing as an intermediate or a building block for other molecules. The name sounds intimidating, but in plain terms, it’s just a small, heterocyclic compound playing a supporting role in much bigger chemical processes.
Handling any laboratory chemical raises safety questions. In my years around chemical storage rooms, an instinct grows — read the Safety Data Sheet, keep those gloves handy, trust the goggles. Piperazin-2-One isn’t different in that respect. Official data show it generally doesn’t pose the same threat as something volatile or highly toxic like concentrated acids or volatile organics. That said, most safety data still recommend standard laboratory gear: nitrile gloves, goggles, and a lab coat.
According to the European Chemicals Agency, Piperazin-2-One doesn’t top hazard lists. It doesn’t have a major reputation for being acutely toxic or environmentally damaging. Still, some sources note that it can cause mild irritation if it gets on skin or in eyes. Anyone who’s spent a sweaty summer pipetting or changing gloves after an unknown spill knows that “mild irritation” becomes much more real the third or fourth time it happens in one day.
I once watched a technician get lazy with eye protection while handling a compound the textbooks called “low hazard.” The result? He spent the afternoon at occupational health, not in a lab chair. Even mild chemicals, with enough exposure, cause trouble. Piperazin-2-One can trigger respiratory irritation if handled in powder form, which means respirators or fume hoods matter if you’re weighing out the substance. Chronic exposure hasn’t been well-studied, and that uncertainty should raise caution flags for anyone handling it every day.
Studies on similar piperazine derivatives sometimes show possible risks to the liver or kidneys after long exposure, so even if one particular version shows minor acute risks, the whole group deserves respect. Chemical handling routines built on habit and respect for the unknown help prevent those silent long-term health issues.
My own afternoons in the lab always felt smoother with clear rules. Simple steps — like never working with open containers outside a ventilated hood, checking that gloves have no tears, and washing up after a shift — keep risks low, even with supposedly mild chemicals like Piperazin-2-One. It makes sense for labs to update their standard protocols whenever a new intermediate shows up, and Piperazin-2-One shouldn’t get a free pass just because the data reads “minimal risk.”
Regulatory agencies set exposure limits for a reason, even if those levels look generous. Anyone in an industrial setting has probably seen posters about reporting spills or accidental contacts. Relying on up-to-date training, using Personal Protective Equipment, and storing chemicals in marked containers goes a long way. In high-production environments or research institutions, management should refresh chemical safety briefings whenever new materials are introduced.
Safer habits in handling Piperazin-2-One also come from honest reporting. If a worker notices odd reactions or symptoms, speaking up lets teams identify new risks, especially since toxicity studies can’t always keep up with how rapidly these compounds get used in research and manufacturing.
Piperazin-2-One looks less hazardous than many of its laboratory cousins, but any chemist worth their salt knows caution pays off. PPE, good ventilation, and strict lab discipline keep accidents from becoming statistics. No single chemical ever justifies getting casual — not even the ones with low hazard ratings.
Piperazin-2-One isn’t just another bottle on the shelf in a lab. This compound has a real footprint in pharmaceutical research and chemical synthesis. Keeping it in good condition matters, as anyone who’s seen a batch go off will understand. Talk to folks in a lab, and they’ll mention how one sloppy storage habit can mean spoiled reagents and ruined results. From my time in a university chemical storeroom, I learned quickly how a minor oversight with temperature or humidity can become a costly mistake.
This compound reacts poorly with moisture and open air. Even a brief exposure can lead to degradation, which messes with everything from purity to safety. Nobody likes working with compromised chemicals, especially when health and results are on the line. More than a few seasoned chemists believe in treating every batch like it’s irreplaceable, since high-quality research often hangs on such details.
The importance of keeping things dry and out of direct light isn’t just a line from an old lab manual. Moisture in the air can slip past a loose cap or faulty seal, setting off hydrolysis. That reduces the effectiveness of Piperazin-2-One and possibly produces toxic byproducts. So, the classic rules hold up: dry containers, secure lids, and minimizing time open to the elements.
A cool, steady temperature works best. Most labs rely on storage between 2-8°C. Sticking something at room temperature might seem easier, but the unpredictability of room conditions can shorten shelf life. It’s like storing milk on the counter—the bottom line is, you’re asking for trouble. Refrigerators marked for chemicals only (not next to anyone’s lunch or field samples) cut down on mix-ups and cross-contamination. When Piperazin-2-One winds up in a fridge meant for food or general purposes, safety standards take a hit.
Labels must show clear information: compound name, concentration, date opened, and hazards. During a busy day, I saw plenty of folks reach for a vial, squint at a half-faded label, and wonder if they’d just grabbed acetic acid instead. Good labeling stops confusion before it starts and supports transparency during audits or inspections.
Having flammable or reactive solvents nearby isn’t just sloppy—it’s dangerous. Piperazin-2-One belongs in a chemical storage cabinet meant for moderate-hazard reactive substances. Organizing related compounds together reduces chances of dangerous accidents. Hazardous waste containers nearby give scientists a way to dispose of spills and residues, reducing harm to people and the environment.
Labs that take the time to update storage protocols and invest in reliable containers protect not just expensive chemicals, but the lives and health of everyone working there. Informal mentorship matters here—a new lab member benefits a lot more from seeing careful storage habits than from reading about them in a thick binder.
Chemical storage doesn’t get headlines, but it’s one of the most important habits in any lab. Choosing the right container—usually amber glass, since light degrades some compounds—keeps Piperazin-2-One stable. Training every lab worker on why these habits matter creates a culture of respect for safety. Instead of memorizing rules for one compound, people see the bigger picture: protection for work, reputation, and each other. I’ve watched labs transform after a single close call—reactive attitudes became proactive routines. That’s how big problems get prevented, even with small daily choices.
Piperazin-2-One doesn’t always make the main headlines in chemistry, but in industrial labs, it grabs attention for good reason. Its structure stands out as a springboard for building more complex molecules. Most folks in the pharmaceutical sector know it for playing a central role in drug design—medications for pain relief or mental health often start with it. Chemists appreciate how easily its core links with other groups, which lets them shape new drug candidates. Market studies show the pharmaceutical space keeps looking for building blocks like Piperazin-2-One to design advanced molecules. In my discussions with R&D teams, they point out that using this compound can speed up development timelines and reduce synthesis steps.
While blockbuster drugs usually take center stage, their roots often come back to molecules like Piperazin-2-One. Medicinal chemists look for scaffolds that lend both stability and reactivity; Piperazin-2-One checks those boxes. Some antibiotics rely on it. Anti-psychotic medications feature it along a chain of molecular rings. Piperazin-2-One can serve as more than just a template—it sometimes goes into treatments for diabetes or even cancer therapies. According to a report from Grand View Research, the global pharmaceutical intermediates market continues to expand, and compounds of this type drive a decent chunk of that growth. Having spoken with formulation experts, I’ve seen firsthand how small changes in building blocks ripple through drug effectiveness and safety.
Beyond medicine, chemical companies commonly turn to Piperazin-2-One when creating specialty materials. It pops up in the making of resins, plastics, and even adhesives, especially where they need durability and precise control over chemical properties. Over the years, I’ve seen specialty chemical firms experiment with it for coatings that resist harsh environments—applications ranging from automotive paints to protective films in electronics. Piperazin-2-One earns its place here due to its compatibility with other chemicals and its performance once set into a polymer chain.
Farms today face a constant battle with pests and plant diseases, and Piperazin-2-One slides into the toolbox as an intermediate for synthesizing certain pesticides and herbicides. The reason comes down to its ring structure, which offers a platform for adding other groups that specifically target unwanted bugs or weeds without breaking down too fast in the environment. Through interviews with agronomists, I’ve learned how the push for more sustainable crop protection keeps driving research into such molecular frameworks. Companies look for solutions that strike a balance between effectiveness and minimal environmental footprint, and Piperazin-2-One often features in early R&D pipelines.
The promise of Piperazin-2-One doesn’t come without questions. Cost of production and sourcing raw materials can hold back wider industrial use. Environmental standards push chemical companies to rethink synthetic methods, especially to lower waste and use greener reagents. In the labs I’ve visited, researchers constantly tinker with new synthesis routes or even biotechnological approaches to manufacture Piperazin-2-One more sustainably. Using renewable feedstocks, recycling solvents, or employing cleaner catalysts could move the industry in a safer direction. In conversations with chemical engineers, I’ve noticed a growing emphasis on safety protocols and transparency—important both for factories and the communities surrounding them.
| Names | |
| Preferred IUPAC name | 2,3-Dihydropyrazin-2-one |
| Pronunciation | /paɪˌpɛrəˈziːn tuː oʊn/ |
| Identifiers | |
| CAS Number | 1193-18-6 |
| 3D model (JSmol) | `3D model (JSmol)` string for **Piperazin-2-One**: ``` CC1CNC(=O)CN1 ``` |
| Beilstein Reference | 113928 |
| ChEBI | CHEBI:38464 |
| ChEMBL | CHEMBL16360 |
| ChemSpider | 1538 |
| DrugBank | DB08230 |
| ECHA InfoCard | ECHA InfoCard: 100.007.701 |
| EC Number | 3.5.2.11 |
| Gmelin Reference | 82249 |
| KEGG | C06314 |
| MeSH | D010888 |
| PubChem CID | 161441 |
| RTECS number | RN0720000 |
| UNII | 6LR8C1B18J |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID8014775 |
| Properties | |
| Chemical formula | C4H8N2O |
| Molar mass | 100.12 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.18 g/cm3 |
| Solubility in water | slightly soluble |
| log P | -1.09 |
| Vapor pressure | 0.000106 mmHg at 25°C |
| Acidity (pKa) | 6.8 |
| Basicity (pKb) | 1.57 |
| Magnetic susceptibility (χ) | -57.0e-6 cm³/mol |
| Refractive index (nD) | 1.558 |
| Viscosity | Viscosity: 2.04 mPa·s (at 25 °C) |
| Dipole moment | 3.23 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 253.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -136.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | Std enthalpy of combustion (ΔcH⦵298) of Piperazin-2-One: **-3202 kJ·mol⁻¹** |
| Pharmacology | |
| ATC code | N05CM18 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 143°C |
| Autoignition temperature | Autoignition temperature: 482°C |
| Lethal dose or concentration | LD50 oral rat 1440 mg/kg |
| LD50 (median dose) | LD50 (median dose) for Piperazin-2-One: "4050 mg/kg (rat, oral) |
| NIOSH | ST6478000 |
| PEL (Permissible) | No established PEL |
| REL (Recommended) | 100 mg |
| Related compounds | |
| Related compounds |
Hydantoin Piperazine 2-Pyrrolidone Glutarimide |