Curiosity about amino acid derivatives in the mid-1900s started to point scientists toward Pidolic Acid, known to some as pyroglutamic acid. This attention came from its role in human metabolism, particularly its presence in brain chemistry and its status as a cyclic derivative of glutamic acid. As synthetic organic chemistry matured, research efforts in both Europe and the United States produced routes to isolate and purify Pidolic Acid, giving it a firmer place in pharmaceutical and nutritional applications. Technical literature from decades ago observed the challenges around stability and sufficient supply, but today’s chemists have a reputation for refining the extraction, crystallization, and quality assurance steps, leading to the variants sold in bulk by biochemical suppliers.
Pidolic Acid, also called 5-oxoproline, makes appearances in clinical nutrition, wellness supplements, and even skin care. Its cyclic structure comes from an internal reaction between the amino and carboxylic groups in glutamic acid, shaping how it behaves in solution and in the body. It has made its way onto consumer shelves often paired with zinc, magnesium, or potassium, riding on research suggesting its role in nutrient absorption and neural function. Pharmaceutical-grade Pidolic Acid tends to reach buyers with strict purity requirements, with ever-more transparent supply chains showing exactly where and how this molecule is sourced and processed.
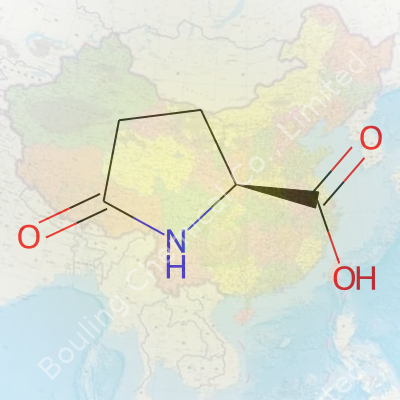
As a white crystalline solid, Pidolic Acid feels almost unremarkable in appearance yet brings distinctive physical properties to the table. Its melting point settles near 150°C. In water, the acid dissolves easily, showing moderate solubility in ethanol, stubborn insolubility in most non-polar solvents, and a mild, acid-like taste. Chemists working hands-on value its zwitterionic character at physiological pH, which means in solution, the molecule carries both positive and negative charges, making it interesting for drug formulation and transport studies. Analytical testing, using NMR, IR, or chromatography, proves that maintaining certain humidity and temperature conditions during storage prevents decomposition and guarantees potency.
Technical data sheets from major chemical providers set Pidolic Acid above 99% purity for most applications, with heavy metal limits below 10 ppm and water content kept under 0.5%. Labels list not only the IUPAC name (5-oxo-L-proline) but also common synonyms such as pyroglutamic acid, ensuring clear identification. A reliable supplier marks every container with precise batch numbers, production date, storage recommendations (generally cool, dry places away from light exposure), and proper hazard pictograms. Product information typically notes compliance with REACH or FDA regulations and includes explicit guidance covering safe handling for industrial and laboratory environments.
Manufacturers mainly turn to two methods: chemical synthesis and enzymatic conversion. Synthetically, cyclization of glutamic acid or its esters in acidic or heated conditions yields Pidolic Acid, demanding carefully controlled reaction times and temperatures to avoid side products. The enzymatic route, often favored for higher purity or “natural source” claims, involves fermenting specific bacterial cultures capable of converting glutamic acid in large tanks, followed by downstream steps of filtration, extraction, and crystallization. For researchers, scaling up brings its headaches—impurities, by-product monitoring, and energy costs—but process optimization through pH adjustment, recycling of solvents, and improved filtration can ease the pains.
Pidolic Acid’s structure attracts chemists eager to test its limits. Amino group protection and deprotection chemistry allows it to serve as a backbone for peptide assembly. In acidic or basic conditions, ring-opening reactions yield glutamic acid, making it valuable for probing degradation pathways in pharmaceuticals. Modifications include alkylation on the nitrogen, esterification at the carboxylic group, and even attachment to metal ions for targeted delivery studies. These reactions aren’t only classroom experiments—they underpin the production of several brain health supplements and investigational medical products. Stability under typical pharmaceutical processing—high heat, mixing, solvent exposure—convinces quality managers of its ruggedness, while batch records help track variants and adapt recipes for niche industries.
Global trade in this compound uses a range of names: pyroglutamic acid, 5-oxo-L-proline, pidolate, and even pidolicum acidum. Pharmacy shelves display “magnesium pidolate,” “potassium pidolate,” and “zinc pidolate,” all based on the same core molecule but with mineral pairings to suit dietary needs. Abstracts from clinical research might mention “oxoproline” or “pyroGlu,” especially in context of biochemical cycle discussions. Recognizing these synonyms proves vital for buyers, regulators, and researchers scanning ingredient lists or patent claims for safety, efficacy, and intellectual property purposes.
Routine industrial handling of Pidolic Acid means built-in checks for dust control, ventilation, and limits on exposure. Occupational safety sheets warn against inhalation of crystals and advise rinse-away procedures for skin and eye contact. Standard operating procedures focus on sealed transfer, wearing safety glasses or gloves, and label verification before use. Large-scale sites often use batch testing against phthalate and heavy metal contamination, logging results for regulatory audits. Disposal rules classify the solid as non-hazardous in most regions, allowing landfill or incineration—but only after checking for tableting excipients or colorants if it comes from processed goods. Transport follows general chemical safety guidelines, with secure containers and emergency numbers in shipping documents.
The reach of Pidolic Acid extends far. Nutritionists turn to its salts — magnesium pidolate and zinc pidolate — for their absorption-friendly profiles. Doctors study its impact on the nervous system, since it affects glutathione synthesis and has links to memory and alertness in older adults. Dermatologists include it in creams or gels, trusting its moisturizing effect and ability to support the skin’s natural protective barrier. Industrial formulators blend it into electrolyte drinks, sports supplements, and sometimes even fortified infant formulas. Laboratory teams value it for biochemical assays, calibration standards, and as a pH buffer. Stories from practitioners sometimes highlight puzzling side effects in sensitive individuals, driving ongoing work to tighten specifications and clear up source traceability.
Current research delves into its neuroprotective potentials, antioxidant qualities, and role in chronic disease management. Academic journals fill with data on its ability to cross the blood-brain barrier, interact with glutamatergic neurotransmission, and participate in the body’s recycling of glutathione. Recent clinical trials look at whether pidolate supplements help with attention, memory retention, and even mild depression. Developments in drug delivery explore combinations with peptide drugs that enhance brain uptake through transporter hijacking strategies. Teams in big pharma balance hopes of new patents with risk management; with every new formulation, questions about stability, patient outcomes, and regulatory hurdles seem to multiply.
Toxicology studies show Pidolic Acid has a high margin of safety at doses used in humans. Animal models tolerate surprisingly large quantities without acute toxicity symptoms. Some individual sensitivities appear—nausea, stomach upset, rare allergic skin rashes—yet cancer or mutagenicity signals have not emerged in long-term testing. Regulatory bodies, including the EFSA and FDA, track clinical reports and batch-test nutritional supplements to keep exposure levels clearly below upper limits. Researchers review metabolic waste products, uncovering that excess Pidolic Acid breaks down rapidly in the kidneys and liver, reducing the risk for buildup in body tissues. Educational materials urge diets that avoid massive ingestion and stress the need for oversight in formulation and labeling, particularly in pediatric or geriatric populations.
I see Pidolic Acid branching out into new therapeutic spaces as science closes in on personalized medicine and cognitive health. Interest is rising in targeting neurotransmitter pathways for psychiatric conditions, where the molecule’s unique chemistry provides both a substrate and a marker for tracking disease stages. Cosmetic industries continue to tap into demand for science-backed ingredients, betting that the moisturizing appeal and barrier support hold strong even under stricter ingredient labeling rules. Producers in Asia and South America respond to upward demand spikes by streamlining fermentation and cutting down on energy inputs, thus lowering the environmental and economic footprint. Research programs tackle the enzyme pathways that generate and recycle Pidolic Acid in the body, hoping that deeper understanding will translate into treatments for neurodegeneration, oxidative stress disorders, or rare metabolic diseases. The path forward points to collaboration between chemists, doctors, and policymakers, ensuring Pidolic Acid carves out a lasting spot in the toolkit of human health and science.
Pidolic acid, sometimes called pyroglutamic acid, comes up often when you dig into nutritional supplements and medicine. It is not one of those trendy compounds you see all over wellness blogs. Pidolic acid lands on my radar mostly due to its connection to supporting brain health and helping with how the body processes proteins and neurotransmitters.
As someone who has watched the supplement world get crowded with all sorts of promises, I see pidolic acid crop up mostly in formulas aimed at cognitive support. It’s no coincidence. This compound plays a role in the cycle that creates glutamate, a neurotransmitter tied closely to memory, learning, and mood. In simple terms, pidolic acid helps keep the gears turning in your brain by making sure there’s enough raw material for vital chemical signals. During times of stress or in aging, the body sometimes struggles to keep up with the demand for neurotransmitters. A few studies point to pidolic acid as a helper to boost cognitive function or restore balance after mental burnout.
Another area where I ran into pidolic acid: protein supplements. It shows up in the ingredient lists of some amino acid complexes for athletes. Here’s the deal—pidolic acid forms naturally as proteins break down in the body. It helps make glutamine and proline, both important amino acids. Glutamine supports muscle recovery and immune balance, which are issues I’ve heard about from people who train hard or push their bodies in tough conditions. There’s talk in some scientific circles about pidolic acid helping the body better absorb and use these amino acids by delivering them in a more bioavailable form.
As a writer who has covered skincare trends, it’s hard to ignore how many ingredients once used in medicine show up in beauty brands. Pidolic acid gets used in some moisturizers and hair care products. It helps the skin hold water—acting as a humectant. Dryness plagues plenty of us, especially in harsh weather or with age. Keeping skin hydrated is not only about comfort but also about keeping the skin barrier strong, so using moisturizers with these types of acids might make a difference for people who struggle with chronic dryness.
Even with these benefits, any compound touted for its health perks deserves scrutiny. Pidolic acid naturally occurs in food and in the body, so moderate use in supplementation carries low risk for most healthy people. There’s limited evidence of harm. Still, research has not yet proven beyond a doubt that extra pidolic acid brings big results for every person, especially over long periods or at high doses. Some reports tie very high levels of it with metabolic issues in people with rare enzyme deficiencies. This underscores the importance of medical oversight, especially for anyone navigating complicated health situations.
Pidolic acid gives us another example of nature’s subtle ways of maintaining the body. We see the best outcomes when we respect the delicate systems at play—ensuring any supplement fits with individual health goals and medical needs. Rather than jumping all-in, most people do better getting quality advice from doctors, especially if they are considering pidolic acid alongside other medications or supplements. Better research will help clarify how this molecule truly fits into preventive and supportive care. Until then, pidolic acid makes its mark as a quiet helper in the background of health and nutrition.
Pidolic acid doesn’t grab headlines very often, but it pops up in supplements and even skincare products. It’s tied to amino acid metabolism and seems to help with cognitive function. Doctors sometimes discuss it in the context of treatments for memory troubles or stress. You’ll find it listed as pyroglutamic acid on some ingredient labels.
People like to believe an ingredient with medical roots will always stay friendly to the body. Pidolic acid mostly keeps a low risk profile, but side effects aren’t out of the question. I’ve read case studies and chatted with pharmacists who’ve seen issues pop up, especially for those taking higher doses or already dealing with kidney or liver challenges.
Some folks have reported headaches, nausea, or digestive discomfort after using supplements with pidolic acid. These complaints don’t always last long, yet anyone managing chronic conditions can see sharper symptoms. Medical journals talk about the rare possibility of confusion or even mood swings, particularly when someone’s using other medications at the same time. High doses have led to acidosis in a handful of cases, a serious drop in blood pH, but this tends to happen mostly with underlying health problems at play.
We have a habit of assuming less common supplements pose little to no danger. People trust health food stores more than they trust prescription bottles, maybe because the packaging feels gentler or the words sound more scientific than threatening. My own family has tried supplements, hoping for a 'natural boost,' without weighing the risk of side effects — especially when mixing several products at once. This optimism can get risky. Regular folks without health training rarely realize how substances interact or build up in the body.
Kidney and liver filters work overtime handling extra amino acids or their derivatives. If these organs aren't performing at their best, even a modest daily dose has the chance to spark reactions. Older adults or patients juggling complex medication routines face double the risk. Even with just one new supplement, their bodies may respond unexpectedly, which has led to hospital visits in several reports. Not every side effect shows up right away, either. Sometimes people brush off headaches or nausea, blaming stress or diet, and only later connect the dots.
Doctors and pharmacists deserve more airtime on these topics. If you’re considering pidolic acid, it pays to talk openly with a healthcare provider. Bring a list of everything you’re taking — prescription meds, over-the-counter pills, herbs, and vitamins. Pharmacists can flag known interactions or highlight early warning signs to watch for.
Patients with compromised kidney or liver function should approach any new supplement with caution. Blood tests can spot hidden metabolic problems before symptoms erupt. Even simple communication, like asking about recent headaches or stomach trouble, keeps the conversation safer and more fact-based.
Independent lab testing of supplements helps weed out bad batches or impure forms of pidolic acid. Look for brands sharing lab results or using third-party verification. While the FDA doesn’t review every new supplement, they’ll report flagged side effects to the public, making it easier for doctors and patients to avoid repeat issues.
Everyone wants lasting health support from vitamins and amino acids. Chasing the latest ingredient won’t fix every problem, and not every supplement’s risk profile matches up with every person’s body. My own advice: ask questions, stay curious, and keep track of how your body reacts. That habit could mean more to your health than the label on a supplement bottle ever will.
Walking into a pharmacy, you might spot unfamiliar names on medicines and supplements. Pidolic acid can look like just another long, scientific word. In reality, this compound often shows up in medical products aimed at supporting the nervous system or bringing balance to amino acid levels. Anyone thinking about using pidolic acid has likely wondered: how should someone take it correctly?
From my years working around clinics and pharmacists, I've learned that no one wants to poke around in dosing charts or squint at fine print while feeling sick. People want clear advice. The key is always: follow the dose your doctor sets, because individual needs can swing based on age, health problems, and what other medicines you take. Taking too much or too little often cancels out any benefit and can even cause harm.
Most of the time, pidolic acid shows up as a capsule or tablet. Water makes swallowing easier and helps absorption in the gut. Doctors, by routine, point out the value of keeping a schedule—taking each dose at the same time every day. Packing doses with a meal can cut down on upset stomach. Skipping food sometimes leads to nausea or discomfort. A full glass of water goes a long way in avoiding dryness or roughness in the throat.
If you miss a dose, there’s no need to double up next time. People fall into this trap thinking more will make things “catch up,” but the truth is, doubling can bring side effects like headache or stomach troubles. Missing a single dose rarely limits long-term results if you just take the next scheduled one as usual.
Doctors study how pidolic acid behaves in the body and shape their dosing advice from real-world experience and careful research. Using too much—for example, more than 1000mg a day for weeks—can lead to dizziness or gut problems, especially for children or pregnant women. The US National Institutes of Health keeps a close watch on dosing research for lesser-known amino acid compounds like this, and regularly updates safety guidance. Trusting those data-backed numbers keeps risk in check.
People who already struggle with kidney troubles, or who take lots of medications, have a greater chance of trouble with amino acid treatments. Sticking with the lowest effective dose is safest, but always let your doctor know every pill or supplement you’re using, even ones sold over the counter.
My work in patient care showed how many folks believe supplements carry little risk. This isn’t true. Products like pidolic acid can upset a delicate balance in the body’s chemistry if taken without medical advice. Relying on tips from friends or information from unverified sources can bring more risk than relief. Supplements interact with prescription medicine, and only a health professional reviews the full picture.
Clear guidance, honest communication with your healthcare team, and sticking to one source for your medication provides real results. Reading instructions before starting any new medicine, and reaching out with questions, keeps everyone safe and helps medicine do what it’s designed to do.
Formulated in labs and found naturally in some foods, pidolic acid shows up on supplement shelves as a form of pyroglutamic acid. Some dietary supplements use it to help with cognitive function or as a component in sports nutrition. A glance at ingredients in energy bars, amino acid blends, or brain health products brings it up now and then.
Stepping into the evidence, most of what scientists know about pidolic acid comes from basic studies. Human trials are few. Some early work focused on how pidolic acid supports the body’s ability to use glutamine, a key amino acid. Researchers found that it plays a part in neurotransmitter cycles, which opens the door to claims about better memory or less fatigue. Over-the-counter products run ahead of the published science, often touting long-term brain or muscle health. But most studies last only a few months at most. Reviews from trustworthy medical sources, such as the National Institutes of Health, do not include pidolic acid on their safe-for-long-term-use lists.
People looking for a supplement that works in the background without much risk usually want to know what happens after a few years. Here, information falls short. Some studies touch on side effects including headaches, insomnia, or digestive issues, but nobody knows if these get worse after long use.
Another point comes up with people living with kidney trouble. Since the body handles many amino acids through the kidneys, those with kidney disease face extra uncertainty. Certain prescription medications can interact in unpredictable ways when paired with pidolic acid because it tweaks glutamate cycles. Without answers from multi-year studies, the best move for anyone with chronic illnesses is to ask a healthcare provider before starting.
During my own time in health reporting, I’ve seen the drive to enhance focus or muscle mass push people to try supplements with short scientific track records. FDA rules mean dietary supplements remain on shelves until somebody proves a safety problem — not before. So, companies set doses and usage timelines on their own. Even substances found in food can show unexpected effects if taken every day in transplant-level amounts. Nutrients work best as a team; single-ingredient megadoses break the balance, and bodies don’t always adjust smoothly.
Cases like omega-3 pills make the risk clear. Decades passed before experts knew that high doses could upset blood clotting in some people. In pidolic acid’s case, time has not yet uncovered the outcome of ten or twenty years of daily intake.
Facing a lack of long-term safety data, scientists and doctors want robust, well-controlled trials. Until data catches up with marketing claims, cautious steps pay off. Reading labels, keeping dosages modest, and having regular check-ins with a doctor keeps risk low. Health decisions, especially ones meant to help over decades, work best with patience and conversation rather than jumping on trends. Meeting dietary needs through varied foods and carefully tracking new research gives people more protection than supplements promising quick benefits with little groundwork.
Pidolic acid, sometimes called pyroglutamic acid, occurs naturally in the body and is used in some dietary supplements. It gets attention in the health field for its role in protein metabolism and cognitive support. Since pidolic acid is tied to amino acid processes, people might assume it's harmless to combine with other substances. Yet, many health supplements or over-the-counter ingredients can deliver more than expected when mixed with prescription drugs.
Think about all the vitamins, supplements, and prescriptions that fill a bathroom cabinet. Even something natural can disrupt how medications work, especially for people managing chronic disease or using complex drug combinations for blood pressure, mental health, or diabetes.
Healthcare professionals sometimes miss supplement use during exams. According to a 2022 review published in the British Journal of Clinical Pharmacology, over 20% of people combining dietary supplements and medications experience some level of interaction or unexpected effect. That number keeps climbing as more folks try to take charge of their health with supplements.
As of now, clinical trials on pidolic acid alone haven’t flagged any widespread, dangerous drug interactions. Most pharmacy databases, including FDA and MedlinePlus, report limited evidence. Yet, just because research hasn’t caught up doesn’t mean problems can’t show up, especially in vulnerable groups like older adults, people with liver or kidney disease, or those taking several prescriptions.
Pidolic acid ties into glutamate metabolism, and that catches the eye of neurologists since drugs for Alzheimer’s, depression, and epilepsy often target glutamate or GABA systems. People receiving medications that affect the brain should always double-check with a pharmacist before adding supplements. A study published by Neurology Research International in 2020 pointed to rare but real risk for people on glutamatergic drugs, including seizure medications and some antipsychotics.
Years ago, a family member dealing with chronic fatigue tried adding amino acid supplements, thinking it couldn’t hurt. She didn’t realize her anemia treatment relied on precise nutrient levels and kidney function, and that not all supplements get processed safely. After some shortness of breath and a routine check, her doctor flagged possible interference. It turned out, one supplement—supposedly “natural”—slightly raised markers the doctor had been watching. This small scare drove home the message that all products, no matter the source, deserve respect in a care plan.
One practical step involves listing every pill, powder, or supplement you take—no matter how minor. Pharmacists can often spot risks that don’t show up in standard doctor visits or electronic charts. For anyone with several medications or chronic health problems, a quick conversation with a pharmacist or nurse can flag trouble before it grows.
Another fix comes from research. Scientists and public health groups need more funding for real-world studies testing supplements in people with common medication routines. Knowledge gaps leave both patients and doctors in the dark, and encouraging open discussion at every appointment helps everyone make safer choices. Staying curious and cautious gets better results than trusting that “natural” always means “harmless.”
| Names | |
| Preferred IUPAC name | (R)-pyrrolidine-2-carboxylic acid |
| Other names |
Pyrrolidone carboxylic acid PCA Pyroglutamic acid 5-Oxoproline |
| Pronunciation | /paɪˈdɒlɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 24598-73-0 |
| 3D model (JSmol) | `3D model (JSmol)` string for **Pidolic Acid** (also known as **Pyroglutamic acid**): ``` C1CC(=O)NC1C(=O)O ``` |
| Beilstein Reference | 91452 |
| ChEBI | CHEBI:18177 |
| ChEMBL | CHEMBL1239 |
| ChemSpider | 2336 |
| DrugBank | DB00111 |
| ECHA InfoCard | 20-20-7550 |
| EC Number | 3.5.1.18 |
| Gmelin Reference | 9697 |
| KEGG | C00624 |
| MeSH | D010866 |
| PubChem CID | 6816 |
| RTECS number | UW8882000 |
| UNII | 5080A8FR9Z |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID2089686 |
| Properties | |
| Chemical formula | C5H7NO3 |
| Molar mass | 129.13 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.485 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.5 |
| Acidity (pKa) | 2.2 |
| Basicity (pKb) | 3.31 |
| Refractive index (nD) | 1.528 |
| Dipole moment | 5.47 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 181.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -818.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1345.4 kJ/mol |
| Pharmacology | |
| ATC code | A16AA07 |
| Hazards | |
| Main hazards | Hazardous if swallowed, may cause eye, skin, and respiratory irritation. |
| GHS labelling | GHS labelling of Pidolic Acid: **"Warning; Exclamation mark; H319: Causes serious eye irritation."** |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry, and well-ventilated place. Avoid contact with eyes, skin, and clothing. Wash thoroughly after handling. Use personal protective equipment as required. Do not breathe dust or fumes. |
| NFPA 704 (fire diamond) | NFPA 704: 1-1-0 |
| Flash point | >140°C |
| Lethal dose or concentration | LD50 (oral, rat): 8130 mg/kg |
| LD50 (median dose) | LD50: 5000 mg/kg (rat, oral) |
| NIOSH | Not established |
| PEL (Permissible) | Not established |
| REL (Recommended) | 30 mg/kg bw |
| Related compounds | |
| Related compounds |
L-Pyroglutamic acid D-Pyroglutamic acid Glutamic acid Glutamine N-Acetylglutamic acid |