Looking back at the start of phenazine’s journey, folks in chemistry labs during the 19th century didn’t care much about complex molecular structures, but they sure noticed when a compound like phenazine could throw out some wild colors. August Lauth reported its isolation in the 1850s, digging phenazine out while he played with coal tar distillation. Instead of letting phenazine fade with the fashion of synthetic dyes, researchers kept poking at it. The late 1800s saw chemists grabbing hold of this aromatic system for all sorts of experiments, sometimes for dyeing, sometimes just to pin down its properties. Even now, someone flipping through the old chemical literature stumbles on how scientists pieced together phenazine’s structure long before NMR or GC-MS showed up, a real lesson in persistence and creative guesswork. Its transformation from a dye to an antibacterial candidate shows that chemistry isn’t just about new technology; the curiosity of people is behind every major step.
In its essence, phenazine is an aromatic ring system, meaning it looks like a pair of fused benzene rings, but with two nitrogen atoms wedged in. Chemists count more than 100 naturally occurring phenazine derivatives, and the variety keeps growing. Today, manufacturers sell phenazine mainly to labs for research — sometimes in fine white to yellow crystals, often in powder. The compound crops up in basic catalogs of chemical suppliers under several forms, from untouched phenazine base to its funny little children, like pyocyanin and neutral red. On the shelf, phenazine promises to spark reactions for those working in microbiology, analytical chemistry, or dye development.
Crystals of phenazine often come out yellow, lending an earthy, almost smoky scent if you happen to get close. It melts around 170°C, a trait useful for anyone planning to purify it. Less welcome, phenazine barely dissolves in water; put a pinch in a test tube and the solution stays stubbornly clear. Drop it into ether, hot alcohol, or benzene, and the stuff dissolves with a bit more cooperation. What makes phenazine worth attention is the way its aromatic backbone invites electrons to shuffle around, making it a solid redox partner. Its chemical stability means it sticks around during synthetic steps, but don’t expect a wild show of reactivity unless you bring in something strong, like acids or halogens, to wake up its nitrogen atoms.
In chemical catalogs and shipment boxes, phenazine gets a CAS number (92-82-0) and usually arrives packed by weight, anywhere from tiny glass vials for research to larger drums for industry. The Safety Data Sheet flags flammability, confirms it should stay away from open flame, and spells out safe handling with gloves and eye protection. In the lab, phenazine goes by its molecular formula C12H8N2, and every package comes labeled with relevant hazard pictograms and purity grades, often not below 98%. Regulatory details differ by country, but any proper chemical supplier tracks it as an irritant — not something to dump down the sink or handle bare-handed.
Anyone aiming to get their hands dirty with phenazine can follow traditional steps or modern shortcuts. The old-school route starts with aniline and nitrobenzene, mixing them and adding a reducing agent, usually in the presence of iron filings and acetic acid, to spark the cyclization that gives phenazine its fused rings. By today’s standards, that method’s a bit messy, so current labs lean on greener oxidants, catalysis, or microwave heating — quicker, more efficient, less chemical waste. Either way, the process winds its way toward crude phenazine, then uses crystallization, washing, and drying to get the clean final product. It’s an exercise in patience and practical chemistry.
One of the great things about phenazine sits in its reactivity. Throw it into a mix with strong acids or oxidizers, and it grabs substituents at the nitrogen positions. Chemists hungry for functionalized building blocks start by attaching alkyl or aryl groups and push the chemistry even further with halogenation, nitration, or sulfonation. The phenazine ring system survives these treatments, making it adaptable both for synthetic dyes and, more recently, for redox-active materials. Those in organic synthesis sometimes twist phenazine into new structures by building derivatives which mimic antibiotics or serve as molecular probes. It’s a playground for those who want to squeeze out new properties from a simple core.
Phenazine isn’t exactly a household name, but it goes by other labels. Older books might call it Azophenylene or even Dibenzopyridazine. The pharmaceutical world, if it ever circles back to natural antibiotics, lists phenazine derivatives under names like pyocyanin, phenazine-1-carboxylic acid, or clofazimine. In color chemistry, it rides under trade names or dye designations, especially when companies patent a particular twist on the parent structure. In purchase orders and supply catalogs though, calling it simply “phenazine,” with the right CAS number, cuts through confusion.
Anyone who’s spent time near an open bottle of phenazine knows it deserves respect. Breathing in phenazine dust can irritate eyes, skin, and even lungs, so a fume hood and good gloves become part of daily routine. Spills aren’t a good look on any benchtop; phenazine leaves persistent stains and calls for cleanup with lots of wipe-downs and solvent. Regulatory advice flags phenazine as harmful if swallowed or inhaled, and disposal must follow hazardous waste regulations. Emergency protocols hang on lab walls showing what to do if exposure happens: rinsing skin with water, seeking medical attention if things get worse. Setting an example counts more than any posted placard—safe handling is a habit, not a speech.
Phenazine pulls work in more corners of science than most folks expect. It grabbed attention first as a dye precursor, making eye-catching reds and yellows for textiles and inks. Medical researchers dug into its derivatives, finding that bacteria like Pseudomonas aeruginosa crank out phenazine compounds naturally, and those compounds show antibacterial and anti-tumor properties in both test tubes and real cells. Agriculture labs study phenazine’s role in soil—certain bacteria use it as a weapon against crop pathogens. Materials scientists tune phenazine for electrochromic devices and organic batteries, capitalizing on its stable redox properties. Even now, folks explore new phenazine hybrids in solar cells and biosensors, hoping for a wider reach beyond its old dye days.
Research labs chase phenazine’s potential on several fronts. Synthetic chemists wrestle with the challenge of building phenazine derivatives faster and cleaner. Biologists look at soil microbes and wonder whether phenazine compounds could protect crops without toxic chemicals. Drug developers track phenazine’s activity against multi-resistant bacteria, wondering how to sidestep its toxicity while keeping its bioactivity strong. As for industry, engineers slot phenazine into new battery electrode materials, searching for safer, more affordable alternatives to lithium. Funding follows wherever phenazine’s synthesis, application, or biological action promises a big leap forward—one success or failure at a time.
Studying phenazine’s toxic qualities reveals a split personality. At low concentrations, phenazine derivatives help certain bacteria compete and outgrow rivals, but at higher doses, these same compounds can damage healthy cells and tissues. Toxicology studies in mice, rats, and cultured cells link phenazine exposure to liver and kidney problems, skin irritation, and oxidative stress. Regulators measure the amounts that cause harm and set exposure limits wherever possible. Researchers keep a cautious optimism; while some phenazine-based drugs turn up in medicine, such as clofazimine, every new application calls for deep dives into how the compound breaks down, lingers, or harms animal or plant life. Anyone working with phenazine in the lab remembers—good intentions don’t shield against side effects.
Looking ahead, phenazine has a lot on its plate. Efforts in sustainable synthesis may cut down on chemical waste and boost yields, making phenazine more affordable and less polluting to produce. Folks pursuing green agriculture see phenazine-producing bacteria as a natural tool to protect crops without harsh pesticides. In pharmaceuticals, the big question hangs over modifying phenazine’s structure to balance antibacterial punch with low toxicity. Battery and electronics developers see promise in phenazine-based materials for flexible, recyclable devices. Every new decade brings fresh uses as the world faces shifting priorities in health, clean energy, and sustainability. Phenazine won’t grab headlines like flashy tech, yet it keeps earning quiet respect from every scientist who sets out to bend it toward another solution.
Talking about phenazine feels like introducing an old friend from college who quietly became more important over time. Though discovered in the 19th century, scientists only started to truly appreciate phenazine’s utility much later. The compound shows up in places that sometimes surprise even folks trained in chemistry.
In nature, phenazine works like a sort of biological defense system. Soil bacteria known as Pseudomonas produce phenazine compounds to keep rivals at bay. These bacteria live lives packed with competition, and phenazine gives them a fighting chance by disturbing competitors on a chemical level. This drama happens way beneath our feet, shaping the health of crops and the richness of soil.
Farmers unknowingly reap the benefits. When these helpful microbes thrive, plants fend off diseases without as much chemical intervention. Crops like wheat and rice, which feed much of the world, grow sturdier roots in phenazine-rich soil. Research published in journals like Applied and Environmental Microbiology shows phenazine compounds naturally reduce root disease, lessening the burden on chemical fungicides. This isn’t just science for labs; it plays out on real fields, season after season.
Hospitals and clinics face a different war: stubborn infections, often from bacteria that laugh off our strongest antibiotics. Researchers dug into phenazine and found it can act both as a villain and a potential hero. Certain strains of Pseudomonas aeruginosa make phenazine derivatives like pyocyanin, which not only give colonies a blue-green tint but also help them resist drugs.
Yet, with careful trimming and modification, scientists turned phenazine into a weapon against disease. Some new drugs built from phenazine show promise as antibiotics or even as cancer fighters, slicing through the armor of tough cells. In the lab, researchers see phenazine cut off the energy of cancer cells or wreck bacterial cell walls. If these findings hold up through patient trials, hospitals might stock shelves with phenazine-laced treatments one day.
Beyond health and farming, phenazine also caught the eyes of engineers. The molecule’s structure lets it transfer electrons smoothly, making it attractive for organic batteries and solar cells. Working on prototypes, some teams blend phenazine into electrodes to extend battery life or improve the capture of sunlight in solar panels. A reliable, cheap battery made with phenazine could help store renewable power better, cutting down on fossil fuel dependence.
Phenazine teaches that nature crafts solutions with layers of complexity humans still work to understand. Finding better ways to boost useful phenazine-producing microbes in soil could lift crop yields without extra chemicals. In healthcare, adapting phenazine’s strengths instead of fighting its harmful forms may unlock treatment for some of medicine’s nastiest infections or toughest cancers. On the tech side, keeping an open mind turns even old molecules into tools for the future.
Phenazine doesn’t show up in regular conversations unless you work in biotech, agriculture, or chemistry. It belongs to a group of nitrogen-containing compounds, coming in several versions, produced naturally by certain bacteria. Farmers and scientists appreciate phenazines for their role in controlling crop diseases, as some of these bacteria keep plant roots safe from harmful fungi. Researchers have also found phenazines in clinical spaces, using their unique chemical structures for experiments and, at times, exploring their antibiotic potential. So far, so good — but the big question sticks around: how safe is this stuff for both humans and the environment?
Phenazine compounds aren’t typically found in common household products. Direct exposure for most people remains unlikely, but agricultural workers and lab scientists sometimes work with concentrated phenazine solutions. From personal experience in biotechnology labs, handling these substances demands proper gloves, ventilation, and a respect for the risks involved. Lab safety sheets, or MSDS, label phenazines as irritants – meaning contact with skin or eyes, or, even worse, inhalation or swallowing, can prompt trouble. Symptoms usually include skin rash or eye irritation, though more severe effects come mostly from hefty, repeated exposures. There is scant evidence for long-term chronic health problems from low-level contact but, as with many synthetic and natural chemicals, regulatory bodies suggest minimizing unnecessary exposure.
When phenazine-derived antibiotics enter the human system intentionally (as part of medical treatments or clinical trials), doctors keep a careful watch for side effects like digestive upset or rare allergies. These reactions aren’t unheard of, but don't crop up with the frequency or intensity seen in other chemical hazards. Direct, routine human contact with raw phenazine still isn’t common in the wider population. Proper handling and use — following occupational safety guidelines — lowers personal risks to manageable levels.
In fields and greenhouses, farmers sometimes put phenazine-producing bacteria in the soil to suppress disease. On the surface, this method appears a natural way to cut down on chemical pesticides, which can harm soil organisms or water systems. The picture gets cloudy if phenazine concentrations build up. Some studies show that high levels could hurt beneficial soil fungi, disrupt nutrient cycles, or stick around longer than desired. In my own gardening experiments, I’ve seen microbial soil amendments work wonders for root health, but I always wonder what happens deeper down the line — especially if powerful compounds start shifting local balances.
Regulators like the EPA in the United States have reviewed phenazine products for agricultural use. So far, findings support their safety when used as directed, without evidence of major toxicity to mammals, birds, or most aquatic creatures at standard field concentrations. Oversight agencies keep tabs on potential buildup in food crops and drinking water. People often worry about the unknown: what happens with continuous, large-scale use, or unexpected weather events that could sweep these compounds into rivers?
Keeping an eye on safety means keeping science in the field and the lab, not just on paper. Random testing alone rarely tells the whole story. Farmers and companies using phenazine-based soil treatments could partner with local universities and community groups to monitor water and soil health, catching any long-term shifts early. Equipment and training aren’t luxuries — they’re basics for anyone handling concentrated phenazine or its analogs. Agricultural safety should matter as much as crop yield.
On the regulatory side, thresholds for acceptable levels in soil and water ought to reflect the latest environmental data, not old studies. Researching low-dose, long-term exposure remains necessary — both for people in close contact and for natural insect, fungi, and plant populations that keep ecosystems ticking. If evidence builds that phenazine or its byproducts linger or accumulate, revising practices, switching to new microbial solutions, or scaling back certain uses must stay on the table.
Public conversations usually lag behind scientific developments. Talking through risks and choices in plain language means communities can better weigh the total impact of phenazine, not just its crop-saving benefits. Safety isn't just a checkbox; it’s an ongoing process that needs attention from everyone involved, not just experts.
Out in the fields, what you can’t see can do a lot of good. Phenazine, a family of colorful compounds made by certain soil bacteria, works like a tiny chemical toolbox for plants. Many farmers may not realize that some helpful bacteria down in the earth produce phenazines that support crops without needing pesticides or fancy machinery.
Farmers deal with a raft of challenges: stubborn fungal diseases, tired soil, crops limping along through drought. Phenazine offers a lifeline. Some strains of Pseudomonas, those little workhorse bacteria, crank out these compounds right around plant roots. In my own backyard garden, I’ve watched tomatoes and beans bounce back from wilt when the soil is healthy and rich with the right microbes. Sure enough, the research lines up: phenazine-producing bacteria keep root diseases in check and foster stronger plants.
The real trick lies in how phenazine-based bacteria soften up threats long before a grower sees symptoms. Fungal invaders and some stubborn bacteria just can’t settle in when phenazine is around—the compound damages their cell membranes, breaking down the walls before those pests can get traction. Real-world field trials show substantial drops in disease. Growers swapping out standard fungicides for phenazine-friendly microbial treatments get not just fewer sick plants, but also better yields. In parts of India and the Midwest, farmers using these bioproducts have harvested healthier wheat and rice, keeping losses lower even when weather whips up more trouble than usual.
The story gets even better when we look at long-term soil health. Many chemical fungicides, sprayed year in and year out, start to wear out their welcome, leaving residues and making life tougher for earthworms and bees. Phenazine-driven approaches support a more balanced microbial community without building up dangerous leftovers. On one Iowa research farm, soils treated with phenazine-producing bacteria sprouted more earthworms and hung onto water longer. More organic matter, fewer erosion problems. That’s a win for the next year’s crop and the one after that.
Still, phenazine is no silver bullet. Not every field supports the same bacteria, and building up healthy communities in abused or compacted soils can take several seasons. There’s also the matter of getting growers to trust a name as odd as phenazine—people want results, but buzzwords and unfamiliar names create hesitation. Sometimes, certain conditions like extreme heat or lousy drainage hold back the good bugs and let the old problems creep back in. Money and patience are both in short supply at planting time, and folks need to see that microbes aren’t snake oil from a glossy brochure.
Community-based field trials, more honest conversations between researchers and growers, and support from local extension offices make a big difference. Bringing phenazine into standard crop rotations works best if farmers can see side-by-side test plots, not just read about them in some scientific journal. More companies are now offering mixed microbial formulations, blending phenazine-producers with other beneficial bugs. These blends handle more than one issue at a time, giving growers a safety net against unpredictable pests and weather.
Many hands—including students, scientists, and practical-minded folks—are getting involved in making soil biology less mysterious. Farmers who take a risk on practices like these often end up teaching the rest of the community what works and what flops. Every time a grower replaces a heavy chemical application with a microbe-based treatment, the land changes just a bit for the better. That kind of progress beats the one-size-fits-all cure every single time.
If you’ve worked in a lab, you know how quickly chaos takes over when chemicals aren’t kept in order. Phenazine brings its own set of practical needs. I’ve watched more than one experimental run get messed up just because someone let their guard down with handling or storage. That’s not something to brush off—phenazine isn’t flour or sugar.
Phenazine stores best in a cool, dark place. An ordinary refrigerator can hold the temperature needed, roughly 2–8°C. Heat messes with a lot of chemicals, and phenazine is no exception. Bring heat into the picture, and you speed up degradation. Light plays a role, too. Direct sunlight doesn’t just fade paint or bleach clothes—it breaks down sensitive substances, including phenazine. That’s why I always suggest amber glass bottles. They shield from unwanted light and protect the integrity of what’s inside.
Exposure changes everything. If a lid isn’t badged on tightly, or the bottle doesn’t provide a proper seal, you risk contamination. I remember seeing a colleague leave a cap loose just once—one error, and the whole batch needed to be tossed. Stick with tightly sealed containers. Glass does a solid job here, outlasting plastic, which can leach or react if left for months.
Label every container clearly. In my first year, a poorly labeled bottle meant “phenazine or not” became a painful guessing game. A decent waterproof label and clear writing save headaches, accidents, and wasted chemicals down the line.
Mixing phenazine with strong oxidizers doesn’t end well. You look at chemical incompatibilities on safety data sheets for a reason—these aren’t recommendations you gloss over. Store phenazine away from acids, oxidizers, or bases. I always set up separate shelves, keeping reactive compounds at arm’s length.
Phenazine can irritate skin, eyes, and the respiratory system. Always use gloves—nitrile tends to work best. Lab coats and safety glasses aren’t there just for show. I’ve seen the aftermath of skipping even one step. A splash in the eye or a spill on skin ruins days and puts health on the line.
If you spill it, clean up right away using absorbent material. Dispose of the waste using local hazardous waste rules, not the regular bin. It’s tempting to cut corners, but one careless action can contaminate the whole workspace.
Moving phenazine around means thinking ahead. Use secure, padded boxes. No one wants a shattered glass bottle leaking in the hallway or, worse, in public transit. In winter, don’t let it freeze by leaving it in an unheated trunk. Take it from someone who once came back to find vials busted from freezing—all it took was a rushed morning and forgetting the basics.
Clear written protocols cut down on mistakes. Regular refresher training and real-life examples work better than a paragraph in an email. Color-coded storage and digital tracking stop confusion before it starts. Some labs use barcode scanning for every in and out—extra initial effort, but it saves time and worry later.
Safety and organization pay off. Whether you’re just starting or have spent years at the bench, respecting phenazine’s storage and handling quirks isn’t just about rules—it’s about keeping people, projects, and results on track.
Phenazine doesn’t get the same kind of press as other industrial chemicals, but it pops up from time to time in conversations about dyes, antibiotics, and even certain soil bacteria. For a long stretch, many people didn’t spare much thought toward safety, especially when chemists worked in less-regulated labs. Now things are different, and more eyes turn toward unexpected risks that could lurk behind everyday science.
Breathing in too much phenazine dust or fumes can cause irritation—and not the shrug-it-off kind, either. Many chemical workers, through sharp lessons, learn that constant sore throats or coughing fits trace back to even low-level exposure at job sites. Splashes can leave a decent burn on the skin, itching that won’t quit, and if you touch your eyes, expect stinging regret and a red face. Problems multiply with prolonged contact. Inhalation especially raises the risk for things like respiratory distress; symptoms may pile up slowly, so the cause isn’t always clear at first.
Epidemiological data from the paint, dye, and pharmaceutical industries doesn’t tell the full story. Phenazine and similar chemicals saw use before strict workplace monitoring, and plenty of long-retired chemists only recall the yellow fingers—ignoring what it meant for their lungs later on. Several animal studies point to organ stress if lab mice or rats get repeated doses. There’s been some discussion around potential mutagenicity; some experiments flagged DNA changes under certain conditions, though there’s little proof of a direct link in people.
Phenazine isn’t only a matter for humans. Labs dump trace amounts in waste streams, and soil bacteria can actually produce certain phenazine compounds. At first glance, natural production might sound like a green light, but even natural chemicals build up to toxic levels. Excess phenazine in soil, for example, stunts the growth of certain plants and disrupts bugs living in the field. In streams, phenazine runoff pushes smaller aquatic life toward trouble; too much exposure makes them act sluggish, or, worse, they just don’t survive.
On job sites and in labs, creating clear labeling and thoughtful storage prevents a lot of accidents. Personal stories from old-school paint mixers to today’s graduate students echo a lesson: neglecting gloves or masks, even for a quick job, ends in regret. Mandatory use of basic protective gear would cut down most of the complaints from skin contact. At research sites, fume hoods and proper ventilation might seem like a hassle, but lung damage usually sneaks up on those who gamble with shortcuts.
Disposal raises fresh questions. Dumping phenazine-rich solutions without neutralization just plants a problem elsewhere. Treatment with oxidizing agents can break down phenazine, shaving off some of that environmental threat. Chemical companies and university labs already share data about what works and what doesn’t. More cross-industry cooperation—sharing these logs, not just the best-sellers—would help small players keep up.
Whenever anyone finds out something new about phenazine’s risks, word should spread fast. Safety data sheets already list the basics, but everyday users still miss warnings or skip updates. Regular training helps, sure, but plain talk—real stories of what goes wrong—hits home better than a stack of rules. Building a culture where people voice their concerns without getting brushed off helps catch problems before anyone pays the price in burned skin or worse.
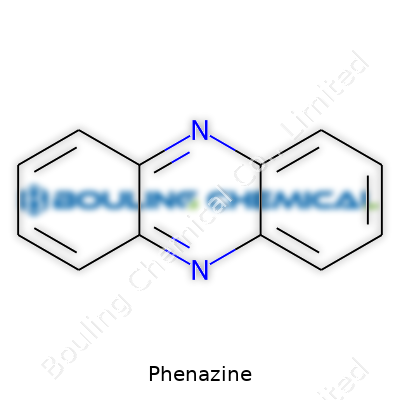
| Names | |
| Preferred IUPAC name | phenazin |
| Other names |
Azophenazine Nitrabine Phenazine base Phenazin 1,4-Diazabenzene |
| Pronunciation | /ˈfiː.nə.ziːn/ |
| Identifiers | |
| CAS Number | 92-82-0 |
| Beilstein Reference | 359699 |
| ChEBI | CHEBI:28851 |
| ChEMBL | CHEMBL1402 |
| ChemSpider | 1176 |
| DrugBank | DB04145 |
| ECHA InfoCard | ECHA InfoCard: 100.003.366 |
| EC Number | 1.10.3.6 |
| Gmelin Reference | 34477 |
| KEGG | C00109 |
| MeSH | D010618 |
| PubChem CID | 4757 |
| RTECS number | SN6475000 |
| UNII | 0W2SX6RXPW |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | The CompTox Dashboard (EPA) for Phenazine is: **"DTXSID7020571"** |
| Properties | |
| Chemical formula | C12H8N2 |
| Molar mass | 180.19 g/mol |
| Appearance | Yellow crystalline solid |
| Odor | Odorless |
| Density | 1.203 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 1.87 |
| Vapor pressure | 0.00298 mmHg at 25°C |
| Acidity (pKa) | -0.6 |
| Basicity (pKb) | -3.74 |
| Magnetic susceptibility (χ) | `-0.59·10⁻⁶ cm³/mol` |
| Refractive index (nD) | 1.644 |
| Viscosity | 1.06 mPa·s (25 °C) |
| Dipole moment | 0.00 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 170.9 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 95.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2975 kJ mol⁻¹ |
| Pharmacology | |
| ATC code | N06DX02 |
| Hazards | |
| Main hazards | May cause eye, skin, and respiratory tract irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06,GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P321, P362+P364, P333+P313, P501 |
| Flash point | 146°C |
| Autoignition temperature | 573 °C |
| Explosive limits | Explosive limits: 1.5–7.0% |
| Lethal dose or concentration | LD50 oral rat 390 mg/kg |
| LD50 (median dose) | LD50 (median dose): 120 mg/kg (oral, rat) |
| NIOSH | PH1990000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.1 mg/m³ |
| IDLH (Immediate danger) | 500 mg/m3 |
| Related compounds | |
| Related compounds |
Azobenzene Phenoxazine Phenothiazine Quinoxaline |