Modern chemistry didn’t spring up overnight, and phenazines played a big role in how the industry grew. Back in the nineteenth century, dye chemists stumbled upon phenazine skeletons as they chased new colors for textile factories. Industrial demand fueled day-and-night shifts in labs as researchers wrangled with the tricky ring systems and hoped some odd molecule might stand out. Phenazine-2,3-diyldiamine grew from this brewing pot of curiosity and practicality. Traditional routes used harsh steps, and chemists leaned on trial and error before the synthetic roadmaps and analytical tools made work smoother today. Early patents and academic papers showed risks, breakthroughs, and the slow, stubborn climb toward something more than a dusty curiosity. This spirit never really left lab culture—curiosity, commercial push, and the promise of new function drive phenazine research even now.
Phenazine-2,3-diyldiamine doesn’t catch the eye like caffeine or aspirin, but its balanced shape—a fused aromatic system with amino groups at two positions—presents unique opportunities. Chemists see its backbone as a foundation for electronic tinkering, since those nitrogen atoms shape how the molecule interacts with both metals and other organics. Many recognize it by names like 2,3-diaminophenazine or the plain “PDD.” Commercial suppliers usually deliver it as an orange to reddish solid, sometimes in bottle-sized portions for anyone from grad students to large polymer workshops. The industry rarely puts it on store shelves for the public, but those who handle specialty chemicals know it well enough—especially laboratories focused on electrochemistry or synthetic biology.
In the world of chemical handling, property sheets tend to look the same, but Phenazine-2,3-diyldiamine asks for more respect than many. The substance holds up with a melting point somewhere in the range of 170 to 200°C depending on purity, and its thermal stability means it doesn’t break down when a reaction demands higher heat. It doesn’t dissolve much in water, forcing chemists to turn to polar aprotic solvents like DMSO or DMF, which can introduce other complications in waste streams or product recovery. A strong, almost acrid smell hits the nose—a reminder not to take its safety lightly. Electrochemical work often focuses on its redox behavior, with reliable cycles that draw researchers looking at battery and sensor prototypes. It’s stable in the dark, but light can push it toward slow degradation, which means it doesn’t fare well in clear bottles under the sun.
Buyers don’t just trust a label reading “Phenazine-2,3-diyldiamine, 99%” anymore. Material safety data sheets (MSDS) break down the hazards—rules call for pictograms showing skin and eye irritation risks and warnings about respiratory protection. Packing usually means tight-sealing amber glass or polymer vials, shielding contents from air and light. Purity requirements depend on the end use, like analytical standards for research chemistry, where every percent of impurity matters. Commercial supply chains pay close attention to REACH or TSCA regulations in Europe or the US, so tracking and documentation follow each package from factory to lab bench. Labeling standards get laid out in black-and-white, with unique identifiers like CAS numbers and batch codes to manage traceability and recall, if ever required.
Synthesizing Phenazine-2,3-diyldiamine builds on decades-old routes, usually starting from readily available precursors like o-phenylenediamine and oxidizing agents. Old-school methods banked on air oxidation, harsh mineral acids, and slow crystallization, but process chemists in well-outfitted labs lean on cleaner, scalable methods today. One practical route brings in nitroaromatic intermediates and controlled reduction steps, slashing byproduct formation. Green chemistry initiatives push for milder conditions, with catalysts or alternative solvents at the heart of the change. Purification means several rounds with column chromatography or even solvent extraction, depending on volume scale. No matter the route, cross-contamination and safety risks—especially from exothermic steps—call for plenty of attention during synthesis.
The basic 2,3-diamino pattern unlocks doors for a whole series of modifications. Chemists keep returning to its strong electron-donating amino groups for building more extended conjugated systems. Electrophilic substitution, N-alkylation, and Suzuki coupling all find space here, with the molecule tolerating physical and chemical tweaks better than many phenazines. Under certain reaction conditions, the amino groups can be acylated, turning the product into a library of new heterocycles meant for dyes or redox chemistry. People working toward coordination compounds use Phenazine-2,3-diyldiamine to tie up transition metals, guiding the shape and reactivity of the resulting complexes. It stands up well to both strong acids and bases, staying useful across a wide pH range.
Those working in chemical purchasing or research recognize a maze of synonyms: 2,3-Diaminophenazine, o-Phenazinediamine, and even regional translations in supplier catalogs. The CAS number (92-61-5) carries more weight than a brand or local label. Some academic papers may call it “DDS-Pz” to distinguish it from related analogs. This spread of names gives headaches to those building databases or comparing literature, but cross-indexing through registry numbers usually clears confusion.
Labs handling Phenazine-2,3-diyldiamine take extra precautions, using gloves, goggles, fume hoods, and sometimes additional respiratory protection if the process generates dust or fumes. Technicians avoid heating it in open air, since decomposition can give off hazardous byproducts. Waste goes in dedicated organic bins, distinct from standard lab trash. Emergency protocols spell out treatments for eye or skin contact, which sometimes results in stubborn staining or dermatitis. Training sessions stress the importance of regular inspection for bottle leaks or age-related degradation, since even sealed vials sometimes develop pressure or an altered chemical profile after storage. Material managers and safety officers keep supply rooms and MSDS databases updated, tracking any signs of regulatory adjustments as research or incidents shift safety perceptions.
Redox-active molecules like this see broad interest across batteries and sensor platforms. Battery researchers value its reversible electron-donating properties and structural rigidity, making it a candidate for next-generation organic flow batteries. Electrodes built from modified phenazines show higher cycle stability, which could reduce costs for scalable energy storage, a puzzle still unsolved at global scale. Dye chemists dig into the molecule’s extended conjugation for use in specialty inks, luminescent probes, or solar cell sensitizers. In biology, its antibacterial roots never faded away, and modified versions find space as probes or biochemical triggers in experimental microbiology setups. Polymer chemistry borrows structure for novel copolymers with predictable conductivity and stability, while environmental scientists notice its persistence and potential for breakdown products with unique ecological effects.
Recent years brought an uptick in papers probing radical cation formation and stability, giving the molecule a spotlight in artificial photosynthesis and catalysis research. Industry and academia both chase modifications that can stabilize multi-electron cycling while keeping synthesis cost-effective. International collaborations stretch from North America to East Asia, pooling modeling, physical testing, and synthetic tweaks. Investment in flow battery startups sometimes highlights derivatives of compounds like Phenazine-2,3-diyldiamine, offering hope for power grids desperate for cheaper, safer energy storage. Pharmachem companies look for ways to turn its antimicrobial strengths into new therapies, though toxicity and selectivity remain big hurdles.
Toxicity research tracks the molecule’s ability to generate reactive oxygen species, which helps explain both its antimicrobial clout and its risks for people. Most studies warn against uncontrolled skin or inhalation exposure, noting cytotoxic effects in mammalian cells after prolonged contact. Animal studies highlight organ-specific effects at higher doses, especially on liver and kidney tissues. Chronic exposure data remain sparse, leaving a gray area for those handling it outside strictly regulated environments. Regulators keep pushing for clearer environmental risk profiles, especially since phenazines can persist in soil and water once released. For anyone tooling with it in the lab, standard personal protective equipment isn’t negotiable, and periodic air quality monitoring stays on the checklist for high-volume processing.
Greater demand for organic materials in electronics and batteries keeps pushing the boundaries on what molecules like Phenazine-2,3-diyldiamine can do. Next-gen battery developers want stable, sustainable materials free from rare metals, and this molecule’s built-in robustness offers a lead. Synthetic methods aimed at greener, less wasteful production could drop costs and improve access. Combination with computer-aided molecule design helps identify new analogs that balance performance and safety, moving beyond what manual screening alone could achieve. Increased transparency around toxicity data, coupled with more responsible sourcing and disposal protocols, will clear the path for broader industrial adoption without trading off human or environmental safety. If energy storage and materials science keep expanding, researchers will keep circling back to this molecule, revisiting old questions and chasing new answers.
Phenazine-2,3-diyldiamine sounds almost unfriendly at first glance—like something you’d find in an industrial drum with a complicated label. Yet behind that mouthful lies a vivid world of science and practical use. I’ve spent years following the trail of unusual substances and tracing how they jump from the laboratory to applications that touch daily life. Phenazine derivatives keep showing up in surprising places, and this one is no exception.
Researchers first turned to phenazine-2,3-diyldiamine because they saw bacteria and fungi do the same. Certain bacteria use phenazine-based molecules as weapons—a chemical edge to hold off competition. Chemists noticed this, then started exploring whether synthetic phenazines could fend off disease in humans. The idea isn’t just wishful thinking. In lab tests, phenazine-2,3-diyldiamine tackles tough patients like Staphylococcus aureus, including strains that ignore regular antibiotics. Researchers signal hope for turning this into topical creams for wounds that stubbornly refuse to heal, especially those stuck with biofilm infections. Wound care teams everywhere constantly face these cases, and better treatments can spare patients months of frustration.
My background includes medical copywriting, so I’ve watched promising compound after promising compound rise…and then disappoint. Still, phenazine compounds fuel new cancer research. They mess with how cancer cells make energy and sometimes halt cell growth altogether. Phenazine-2,3-diyldiamine isn’t a headline oncology drug yet, but there’s a steady hum of interest in universities and pharma companies. Every so often, an obscure compound like this winds up changing how doctors see a type of disease. The pipeline takes years, but it’s how most new medicines finally arrive in pharmacies.
Lab work with phenazine-2,3-diyldiamine rarely stops with medicine. This molecule shows promise as a part of energy storage systems, especially in redox flow batteries. That matters more than ever as industries and cities try to store renewable energy like solar and wind. Traditional batteries run into scaling and safety problems; compounds like phenazine-2,3-diyldiamine might fill a vital gap. It stands up to many charge-discharge cycles, allows energy grids to balance supply and demand, and could make large-scale clean energy storage cheaper. From my time consulting with engineers on energy projects, I’ve noticed real excitement whenever new battery chemicals show up. Real-world testing takes the romance out of things, but this one keeps turning heads.
Chemicals with promise also bring challenges. Toxicity sits high on the list, both for humans and the environment. In the rush to try new antibiotics or fuel cells, it’s easy to overlook disposal, long-term exposure, or side effects. I remember seeing early synthetic dyes treated as harmless until decades of handling them revealed trouble. Researchers need to test toxicity thoroughly and regulators must keep watchful eyes on large-scale manufacturing. No shortcut here—safety and persistence must win every time.
Innovation rides on curiosity and need. Each time scientists spot a molecule that solves a few of today’s headaches—be it infection, cancer, or energy storage—they chase it down until it either disappoints or reshapes an industry. Phenazine-2,3-diyldiamine stands at this crossroads right now. Careful research could push it out beyond the lab, into the hands of doctors and engineers. Its future depends on relentless testing, open collaboration, and sometimes just old-fashioned luck.
Some molecules carry a kind of curiosity. Phenazine-2,3-diyldiamine—try saying that five times fast—is one of those odd bits of chemistry that grab attention. I remember sifting through shelves lined with glass-stoppered bottles, labels peeling off in lab basements, chasing down the story behind molecules like this one. The thrill wasn’t in the name or the dusty bottle. It came from learning how its structure gives it an edge in certain fields, how it interacts with the world because of how its atoms are stacked.
Phenazine-2,3-diyldiamine takes the form of a yellowish solid at room temperature, though sometimes the color leans a bit brown. Holding that powder in gloved hands some years ago brought a slight, earthy, almost faintly medicinal smell—certainly no rose garden. It packs itself tightly thanks to its planar, rigid core, creating dense crystals. Heat it up and it hangs on to solid life up into the 200-degree Celsius range before melting— a pretty stable hand in a world where molecules can fall apart easily.
The molecule dissolves well in organic solvents like ethanol and dimethylformamide. Water hardly budges it, so if you spill a pinch next to the sink, it clings stubbornly to glassware until you bring in something nonpolar. That resistance to water percolates through its uses: not every compound can thumb its nose at a splash.
At the heart of phenazine-2,3-diyldiamine sits a fused ring system with two nitrogen atoms baked in, and those extra two amine (NH2) groups at the 2 and 3 positions make it even more reactive—almost like extra arms reaching out to build new bonds. That means it reacts readily in the lab, eager to link up with acids, metals, or carbonyl compounds. Chemists who work with dyes or who want to tweak electronic properties in new molecules get a lift from these reactive pinch points.
Something surprising about phenazine skeletons is how they can shuttle electrons back and forth. Redox activity isn’t just lab jargon—this quality supports applications in battery research and sensors. The amine arms make this molecule even more helpful, adding nuance to how charges move. I’ve watched colleagues test solutions of this stuff, tapping electrodes in and watching color shifts and current spikes on their data readouts.
Take the way phenazine-2,3-diyldiamine shrugs off water but dances in organic solvents. This opens doors for it to slip into plastics or specialty coatings where water-resistance means longer life and fewer breakdowns. Fabric tinting and biological stains also use phenazine-style molecules; their punchy colors give lasting vibrancy. Back in my graduate days, I used compounds like this to make thin films under UV lamps, hunting for that color shift that says the chemistry is right.
Working with aromatic amines means handling hazards—phenazine-2,3-diyldiamine included. Its stability doesn’t mean it’s risk-free. The amine groups do bring up health and environmental concerns, since some related chemicals slip through conventional treatment filters and linger. Labs and industries can’t just rely on gloves and fume hoods; they need better disposal standards and more selective capture methods for amine-containing waste. Green chemistry pushes for new ways to build molecules like this without releasing problematic byproducts.
Efforts to make derivatives safer and tune them for specific needs—think medicine, sensors, or catalysts—rest on fundamental research. Analytical chemists and synthetic teams can focus on controlling emissions, designing molecules for easy recycling, and supporting data transparency for safer handling guides. Even the old trick of modular chemistry, where pieces are snapped together like Legos, can help reduce toxic byproduct formation.
Phenazine-2,3-diyldiamine doesn’t belong on an open shelf mixed with odds and ends. Too many people skip over the instructions and regret it later. I remember that time an old colleague stacked some reactive chemicals right next to each other to save space. He ended up with a gnarly spill that left a heavy chemical odor hanging in the air for hours. Phenazine-2,3-diyldiamine deserves respect, and it doesn’t take much to keep problems away.
Keep it dry. This compound draws problems when water creeps in. Fluctuating room humidity can change how any powder or crystalline solid behaves, and moisture may cause clumping or unwanted reactions. Every time I work with similar compounds during hot, muggy months, I use a desiccator or sealed container. It only takes one careless habit—like leaving a jar open during a humid afternoon—to lose an entire batch.
Store it away from sunlight. Light, especially ultraviolet rays, can trigger chemical changes in aromatic compounds. Even a few hours of exposure during a summer day can degrade sensitive materials. Amber bottles give an extra layer of protection, and if you store them in a metal or opaque cabinet, odds get even better. Heat also acts as an enemy. If you leave the bottle on a windowsill or near machines that kick out heat, degradation picks up speed. A dedicated chemical fridge (not shared with food) helps keep it steady, ideally at a cool and constant temperature.
Phenazine-2,3-diyldiamine doesn’t always play nice with oxidizers. If you keep it next to bleach, hydrogen peroxide, or nitric acid, you're one spark or spill away from a real mess. I saw an accident years ago where a storage mix-up caused more paperwork and cleaning than actual injury, but it could have gone the other way. Use clear labels, invest in a chemical storage chart, and update your inventory often—don’t let containers gather dust unread behind a row of bottles.
This isn’t just about gloves and goggles, even if those are your first line of defense. Laboratory coats and proper ventilation matter, too. I learned early to stand back, open the lid away from my face, and keep everything at bench level. If a spill happens, containment pads or neutralizers help, but a good plan makes spills rare. Someone always thinks it’s faster to skip the mask “just this once,” but airborne dust isn’t forgiving.
When disposal time arrives, dumping Phenazine-2,3-diyldiamine down the sink is out of the question. Local and national regulations call for separate collection and specialized waste handlers. Not all labs have disposal contracts, so check ahead before buying more than you need. I’ve made a habit of ordering only in small, workable amounts to cut down on leftovers sitting idle and waiting for disposal costs to spike.
It helps to keep checklists near your storage shelves and run monthly safety audits. Training should go beyond paperwork; real examples and stories, including mistakes, stick better. Posting up-to-date emergency contacts, and making sure everyone knows where the spill kits live, actually changes outcomes. Chemical safety isn’t just about avoiding fines—it keeps projects on track and people healthy.
Phenazine-2,3-diyldiamine doesn’t show up in most household pantries, but it does have a clear place in certain research labs and industries. This compound, known for its two amine groups attached to a phenazine structure, gets attention for some interesting chemical properties. At the same time, many people rightly ask if it brings any risks—especially toxicity or hazards worth caring about.
Public data on the safety of phenazine-2,3-diyldiamine don’t stack up to what you find with common industrial chemicals. Researchers have explored phenazine or its derivatives for dye production, bacterial studies, and even in some battery research. With many chemicals, small changes in structure can turn a safe material into something dangerous. The addition of two amine groups to the base phenazine ring might increase the chance for allergies or harmful reactions in people. I remember working on similar chemicals years ago in the lab—gloves, goggles, the full safety gear—and for good reason.
Looking into toxicological databases, the studies on phenazine-2,3-diyldiamine itself are quite thin. That doesn’t mean it’s safe by default. Fact is, other phenazine compounds have flagged issues for skin and respiratory irritation. A few have shown mutations in lab settings—hardly reassuring, especially when thinking about the risks that come with handling powerfully reactive chemicals.
Chemistry labs still see phenazine compounds used, often under tightly controlled conditions. The biggest risk comes from dust, powder, or mishandling the solid form. Inhalation or skin contact rank among likely exposure routes. If an accident happens, these aren’t the sorts of chemicals you want getting in your eyes or yours lungs. For people outside a lab, the chance of exposure stays extremely low—phenazine-2,3-diyldiamine doesn’t make its way into food or public spaces.
The story repeats itself across countless chemicals. Too often, users put trust in products or substances just because they seem obscure or carry a complex name. But with phenazine derivatives, it’s important to remember: little research often means few confirmed effects, good or bad. That kind of uncertainty paints a big question mark over daily handling.As someone who has handled other hazardous organic powders, I’d never open a new bottle of any poorly documented chemical without at least a dust mask and a nearby eyewash station. Even if something seems safe, that’s a gamble not worth taking.
If you end up working around phenazine-2,3-diyldiamine, check material safety data sheets and pay close attention to warnings—though some may be only based on similar compounds. Use gloves, a respirator, and splash protection. Don’t work alone. Make sure ventilation actually pulls fumes away, not toward you. Just like with any chemical that’s not well studied, treat it as more hazardous than its cousin compounds, not less. Any hint of burning, itching, or cough means it’s time to step away and seek help. Cleanup matters just as much; never sweep up dust with a dry broom or leave powder spills sitting out. If you’re uncertain, ask the experts—it’s safer than finding out the hard way.
Ask around in any research lab about tough-to-find chemicals, and someone will bring up Phenazine-2,3-Diyldiamine. This compound isn’t chilling on the shelf at your local hardware store or hiding behind the pharmacy counter. For many, that’s enough to spark a half-hearted search, but for folks determined to dig this chemical up, there are a few things worth knowing.
Big chemical suppliers run their business with both eyes on the law. Sigma-Aldrich, TCI, Alfa Aesar, and the rest of that crowd traffic nearly every compound you’d find in a thick chemistry textbook. Still, Phenazine-2,3-Diyldiamine doesn’t pop up on their easy-to-browse catalogs. That points to a distinction: some chemicals simply don’t fit the high-demand mold. The people who want this stuff aren’t making up a huge market, so suppliers shy away from keeping stock on hand.
My own work in a university chemistry lab taught me that supply margins run razor thin for lots of specialty reagents. Bulk suppliers respond to demand, but if most customers only call once every decade, those requests get back-burnered. On top of that, some chemicals raise compliance flags due to safety, regulatory, or security concerns. That means sellers need an extra stack of paperwork, tighter storage, and sometimes they just say ‘no thanks’ rather than dealing with the hassle.
A lot of university labs hit a roadblock searching for obscure molecules. Purchasing departments start with the big suppliers. No luck? Then they call up smaller regional distributors. In some cases, no listing comes up at all. That’s where custom synthesis comes into play. For those with solid paperwork and an institutional green light, specialty companies will make compounds to spec. Custom synthesis eats both time and budget, and sometimes an email exchange with the right scientist at another institution can uncover a shareable stash tucked in an academic freezer.
Labs with connections sometimes work out informal swaps. A research friend in Germany once mailed a vial across borders, padding the box with a handwritten note in place of official paperwork. Not every lab plays it safe, but nobody wants to get caught dodging import controls or breaking transport rules, so most stick to approved channels and try hard not to cut corners.
For those chasing Phenazine-2,3-Diyldiamine, tapping into professional networks can help. Online chemistry boards and organizations (like the American Chemical Society) boost your odds of finding someone willing to help. Another smart move? Asking custom synthesis outfits for a quote—with enough interest pooled from a handful of labs, pricing drops and turnaround speeds up. In the long run, industry needs to recognize how keeping these specialty reagents available enables small labs and independent researchers to keep pushing science forward.
Hunting for rare reagents never follows a predictable path. Sometimes you strike gold with a supplier willing to take a special order. Sometimes old-fashioned collaboration does the trick. Either way, patience, paperwork, and a healthy address book often beat luck or shortcuts. For students and early-career researchers, leaning on their mentors’ networks can turn up hard-to-find materials—and sometimes, a good story about what it takes to keep discovery alive.
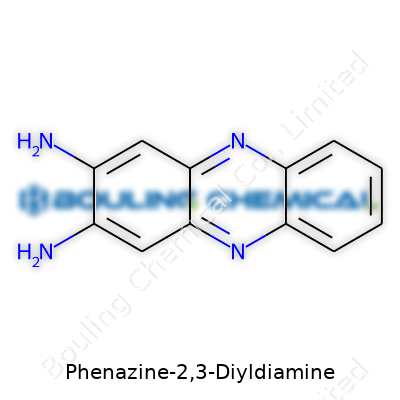
| Names | |
| Preferred IUPAC name | 5,10-diaminophenazine |
| Other names |
2,3-Diaminophenazine Phenazine-2,3-diamine |
| Pronunciation | /fɛˈnæz.iːn tuː θriː daɪˈɪlˌdaɪ.əˌmiːn/ |
| Identifiers | |
| CAS Number | 522-57-4 |
| 3D model (JSmol) | `3D Model (JSmol) String for Phenazine-2,3-diyldiamine:` `NC1=NC2=CC=CC=C2C(=N1)N` |
| Beilstein Reference | **158524** |
| ChEBI | CHEBI:53361 |
| ChEMBL | CHEMBL2106008 |
| ChemSpider | 141380 |
| DrugBank | DB08474 |
| ECHA InfoCard | echa-infocard-en-02d1e948-667f-4cd2-a500-cae0c47e6416 |
| EC Number | 212-692-2 |
| Gmelin Reference | 82850 |
| KEGG | C08373 |
| MeSH | D017957 |
| PubChem CID | 185032 |
| RTECS number | SJ8575000 |
| UNII | E2F6O1T4RE |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C12H10N4 |
| Molar mass | 210.240 g/mol |
| Appearance | Red to dark brown solid |
| Odor | Odorless |
| Density | 1.31 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | 0.33 |
| Vapor pressure | 0.0126 mmHg at 25°C |
| Acidity (pKa) | 4.73 |
| Basicity (pKb) | 12.09 |
| Magnetic susceptibility (χ) | -0.0009 cm³/mol |
| Refractive index (nD) | 1.7400 |
| Dipole moment | 3.54 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 122.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 185.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -408 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | D08AX |
| Hazards | |
| Main hazards | Harmful if swallowed or in contact with skin. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS06,GHS08 |
| Signal word | Warning |
| Hazard statements | H302 + H312 + H332: Harmful if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | P261, P264, P270, P271, P272, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P308+P313, P312, P321, P330, P332+P313, P337+P313, P362+P364, P405, P501 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | 147°C |
| Lethal dose or concentration | LD50 (oral, rat): 300 mg/kg |
| LD50 (median dose) | LD50 (median dose) = 640 mg/kg (rat, oral) |
| NIOSH | KN6475000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.1 mg/m3 |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Phenazine DPPD (N-Phenylenediamine) Phenylenediamine Diaminophenazine |