Chemistry has a way of drawing lines between discoveries, letting one curiosity connect to the next. Phenazine-2,3-diamine comes out of this same story. Chemists began exploring the maze of phenazine-based compounds in the late 19th century, fascinated by the vivid colors and unusual reactivity. As the years ticked by, researchers found that by shifting amine groups onto the phenazine core, especially at the 2 and 3 positions, they could push new boundaries in dye chemistry and pharmaceuticals. Early work focused on colorants and bactericidal agents, but as the century turned, the scientific conversation shifted toward synthetic techniques and structure-activity relationships. Memories of flipping through dog-eared chemical catalogs in grad school come to mind, spotting these names and scribbling notes about their relevance to pharmaceuticals and organic semiconductors.
Stepping into a chemical storeroom, one can find Phenazine-2,3-diamine tucked among bottles of aromatic diamines and specialty intermediates. Chemically cataloged as C12H10N4, this compound stands out due to its two amine groups sitting right next to each other on the phenazine ring. Some know it as 2,3-diaminophenazine, a direct way to remember the locations. You might also spot other names like Phenazine O-diamine or 2,3-PDA in specialist papers and supplier websites. It's not an item you’ll see on a hardware store shelf, but among those working with dyes, sensors, antimicrobial agents, and advanced polymers, it’s a familiar sight.
Touching on its physical character, Phenazine-2,3-diamine takes shape as a fine, yellow to brown crystalline powder. It’s not the kind that dissolves easily in water, but it stirs into organic solvents with a bit of coaxing, especially hot ethanol or DMF. Its melting point sits in the neighborhood of 290°C, which means it demands respect under a laboratory torch. Chemists drawn to aromatic amines will recognize its moderate basicity and a tendency for slow oxidation in air, sometimes leaving stubborn brown residues on lab glassware. Its resonance-stabilized aromatic framework makes it both sturdy and versatile, offering a robust skeleton for further chemical change.
Suppliers package this compound with purity often above 98%, sometimes even 99% for high-performance tasks. Labels feature the CAS number 492-11-1, molecular mass close to 210.2 g/mol, and hazard statements warning about skin and respiratory irritation. Labels frequently warn of possible toxic effects, reminders of the respect needed in handling such materials. One memory stands out of a colleague misplacing a safety label and the amount of time spent retracing steps just to confirm we kept everything safe and compliant.
The classic synthesis involves steps that many organic chemists have come to appreciate for their reliability. One common approach starts with phenazine, sends it through nitration to add nitro groups at the 2 and 3 positions, then employs catalytic hydrogenation to switch those groups over to amines. Each reaction needs attention—over-reduction ruins the aromatic ring, while incomplete hydrogenation leaves the product underfunctionalized. Alternative routes sometimes begin from o-phenylenediamine, looping in various condensation and oxidation steps to close the phenazine core. Every graduate student who’s spent a night monitoring these reactions knows about the careful balance of temperature, pH, and slow crystallization that pays off with a good yield and clean product, especially for use in research labs.
With two free amine groups sitting on a reactive aromatic framework, Phenazine-2,3-diamine loves to take part in further reactions. Acylation, sulfonation, and azo-coupling are just the beginning. In the lab, chemists use this compound to assemble polymer chains, attach it onto surfaces for sensing devices, or introduce further substitutions for targeted medicinal chemistry. Watching it change color as new groups attach offers a real sense of satisfaction. Reactions with aldehydes yield Schiff bases, which turn up everywhere from metal ion sensors to anti-tubercular drug screens. Small changes in functional groups open doors to a surprising range of applications, keeping researchers on their toes and always searching for better results.
Names stack up for a reason. Researchers, manufacturers, and suppliers all carry their own shorthand. Besides Phenazine-2,3-diamine, you’ll come across 2,3-diaminophenazine, perhaps less often as O-diaminophenazine or 2,3-PDA. Different chemical databases also link it to other trade names depending on application, but all roads trace back to the same substance. Each name echoes a different use or synthetic route, so cross-referencing them has become a normal part of double-checking chemical inventories.
Handling Phenazine-2,3-diamine means thinking about more than just gloves and goggles. It’s a strong skin and eye irritant, known to provoke allergic reactions in some individuals. Inhalation of dust poses risks, particularly in poorly ventilated labs. Proper storage conditions call for a cool, dark cabinet away from incompatible oxidizers, with spill procedures lined up for unexpected accidents. I once trained a junior chemist on phenazines; seeing the learning curve with containment, waste disposal, and emergency procedures left a lasting impression. Regular safety data sheet (SDS) review, ventilation checks, and spill drills keep the risk down. In larger-scale environments, fume hoods, dust masks, and chemical-resistant aprons become everyday gear. Disposal routes direct contaminated materials to specialized hazardous waste streams, blocking them from entering general lab trash.
This compound finds a home across many fields, connecting fundamental chemistry to real-world solutions. Dye-makers tap into its oxidation states for creating deep, stable colorants. Medicinal chemists explore its antimicrobial, antitumor, and antioxidant activity. Materials scientists build it into conductive polymers and organic semiconductors, chasing new ways to improve sensor technology or boost solar cell performance. A former colleague used it in electrode surface modification to tweak sensor sensitivity, opening pathways for trace pollutant detection. Its flexibility helps researchers develop unique imaging agents, biosensors, and corrosion-resistant coatings. With environmental science focusing more on toxic metals, Phenazine-2,3-diamine-based sensors stand out for selective metal ion detection in water samples.
Recent years have brought a flurry of research papers trying out new uses for this molecule. Teams in synthetic chemistry keep shifting amine groups, attaching new fragments for targeting pathogens or light-responsive materials. Medicinal chemists find the structure ripe for derivatization, testing analogs for improved bioactivity and less toxicity. Research groups examine polymer films made with Phenazine-2,3-diamine, hoping to match or beat the electrical performance of pricey commercial materials. Funding agencies in several countries have recognized the role of phenazine derivatives in fighting antibiotic resistance, triggering projects that design custom molecules for multi-functional use. One thing stands out: as analytical techniques get sharper, studies focus on minute changes in molecular structure that lead to big shifts in activity, making this compound a chemical playground.
Toxicity always stands as a gatekeeper between lab curiosity and commercial product. Studies show Phenazine-2,3-diamine can irritate skin, eyes, and lungs, so safe handling starts with reliable ventilation and personal protection. In animals, high doses disrupt liver and kidney function. Cell studies point to cytotoxic effects, which catch the attention of both industrial safety officers and pharmaceutical developers. Chronic exposure claims more than its share of cautionary notes in regulatory documents, echoing the stories told at safety seminars about chemists who let their guard down. Toxicity evaluation builds a foundation for smart risk management, steering researchers toward greener derivatives and safer handling protocols.
Peering down the road, Phenazine-2,3-diamine holds promise for new sensors, drugs, and functional materials. Collaborations between chemistry and biomedical engineering keep churning out patent applications for point-of-care devices and next-generation dyes. As the search for antibiotics and smart materials continues, tweaks to this molecule’s structure could tip the balance toward better performance and fewer side effects. New synthesis routes aim to cut waste, lower cost, and use greener reagents. If material scientists can balance conductivity, processability, and biocompatibility, we may see this compound at the core of niche electronics and medical diagnostics. From personal experience in research collaboration, the hunt for stability and safety will always guide decision-making for translating lab experiments into useful products. The story isn’t over; Phenazine-2,3-diamine’s chapters keep getting written in new labs around the world.
Phenazine-2,3-diamine is not a name that pops up at a dinner table discussion, unless someone invites a chemist. The core of this molecule grabs attention through its dual character. Think of it as a fused ring structure — two benzene rings stitched together at an angle, shaping a rigid skeleton. Each ring shares a pair of carbon atoms with the other, forming a system known as phenazine. At the 2 and 3 positions of this core ring, two amine groups (-NH2) dangle like tiny, reactive hands. That’s what earns it the “2,3-diamine” title.
In plain chemistry, the structural formula looks like C12H10N4. The main backbone sets the chemical behavior: flat, aromatic, and electron-rich. Some folks describe this kind of structure as “planar and conjugated,” so it’s no surprise that phenazine-2,3-diamine can interact with many other molecules.
I used to think small organic compounds all blended together in labs, but experience in graduate school taught me each ringed structure offers something unique. In research, phenazine derivatives step out from the crowd thanks to their stability and bright colors. The diamine substitution at the 2 and 3 positions changes everything. Those amine groups can link up with metals, accept or donate electrons, or fit into larger molecules. Thanks to these features, chemists study compounds like this as puzzle pieces for new drugs and even dyes for solar cells.
It’s easy to appreciate how simple changes in chemical design transform an inert powder into something buzzing with potential. Science history offers plenty of examples where a molecule’s nuance led to big breakthroughs—the dye industry in the 1800s and modern medical imaging, both moved forward thanks to compounds built on backbones like phenazine.
Every time someone crafts a new molecule, questions roll in. Can it harm people? Does it break down easily in water? Lab stories often remind me of the caution scientists use with aromatic amines, which sometimes turn out to be tricky for biological systems. There’s always a watchful eye on toxicity and environmental impact.
The flipside shines through the sheer promise of these diamine compounds. Researchers chase after new antibiotics and anticancer agents. Bacteria naturally produce some phenazines to fight off competition. Mix in clever design tweaks—like sticking those amine groups on the 2 and 3 spots—and the molecule starts acting in fresh ways. Synthetic chemists can then use these amines as hooks to join other useful groups, building bigger, bolder molecules.
Stories about new chemicals hardly ever end at their structure. Once a substance like phenazine-2,3-diamine appears, the next chapter always involves digging into the practical details: how to make it safer, how to use it smarter, how to dispose of it without harm. The science community keeps conversations open about alternative synthesis routes that cut out toxic starting materials. Honest discussions among chemists can spark better manufacturing practices, like using solvents that don’t pollute waterways.
One thing stands out after years of reading and lab work: a simple ring and a couple of reactive sites can open a world of uses, but only if chemists keep a keen eye on real-world impacts. Balancing creativity in the lab with responsibility outside it feels just as important as drawing a neat chemical structure.
Phenazine-2,3-Diamine rarely stirs up public chatter, but this compound carries real weight in the world of science and industry. I learned about it working on bacterial research projects where we explored pigment-producing microbes. It surprised me how many branches of work this compound touches—medicine, agriculture, and electronics, to name a few.
Antibiotic resistance continues to outpace new drug development, especially with hospital-acquired infections. Phenazine-2,3-Diamine steps into the spotlight here. Studies show its structure helps scientists build drugs that hit tough bacteria by generating reactive oxygen species—cells find it hard to fend off this kind of attack. Some research also points to its potential for fighting cancer. Cancer cells, living with abnormal redox chemistry, often crumble under the stress caused by phenazine-based compounds. Labs continue to test this, but the blueprint looks promising.
Going beyond medicine, phenazine derivatives spring up in crop fields. Soil microbes churn out natural phenazines to keep out rival fungi and bacteria. Farmers and agri-specialists tap into this by promoting beneficial microbes that pump out phenazine-2,3-diamine. The crops end up stronger, the soil gets a health boost, and chemical pesticide use drops—a win for sustainable farming. I remember an older farmer telling me he’d rather trust the soil’s own defense than keep buying new sprays every season. Stories from the field reflect what research papers confirm.
Energy storage and electronics rely on materials with flexible charge properties. Phenazine-2,3-diamine brings a punch to new battery prototypes. Its core structure allows electron shuttling, acting as a backbone for redox flow batteries. These batteries store energy from renewable sources, something grid engineers talk about with a mix of hope and frustration. Conventional batteries carry high costs and environmental baggage. Compounds like phenazine-2,3-diamine could lower the price and boost reliability. Some teams work on integrating it into flexible electronics, breaking away from rare metals and toxic components. This shift opens doors in consumer tech, medicine, and even off-grid power in developing areas.
New applications don’t arrive without hurdles. Producing phenazine-2,3-diamine on a larger scale means handling safety precautions—its building blocks come with toxic risks, especially for factory staff. Regulations need to catch up, focusing on safe synthesis and disposal. In research labs, contamination or overuse can disrupt microbial systems. Farmers must balance soil health and biodiversity against crop yield boosts.
Striking that balance isn’t a solo job. It calls for honest communication between scientists, regulators, and those who use these products day to day. Grants can help small labs improve safety in pilot projects, and agricultural extension services can fill in knowledge gaps among growers. Industries that want to use this chemical for greener power should share data on environmental effects, not just profits. Workers at every step deserve to know how these new materials shape their work and health. I've sat in quiet meetings between researchers and community leaders—progress comes slow, choices matter, and nobody benefits from skipping questions.
Phenazine-2,3-diamine brings out the strange teamwork between chemistry and biology. Its use in fighting disease, defending crops, and powering technology reflects deep curiosity and sweat from thousands of people. Each step toward mainstream adoption raises new challenges about responsibility and safety. The questions aren’t just technical—they’re about trust, experience, and finding steady ground as new options show up.
A question about the purity or grade of Phenazine-2,3-diamine points straight at what matters most in any hands-on chemistry, whether it's developing new medicines, tweaking catalyst performance, or pushing forward with battery research. Anyone who has set up an experiment or processed chemicals in a lab knows how things fall apart fast when impurities creep in. The smallest bit of contamination turns what should be a crisp and predictable reaction into something messy, unpredictable, and sometimes plain useless.
On paper, technical data might mention phrases like "98% pure" or "analytical grade," but the real value shows up only through trouble-free results. I once spent a week trying to confirm a clean yield with a sample that claimed high purity. My results ran off the rails. Only by switching to a sample with independent supplier verification—complete with HPLC, NMR, and detailed certificates—did I finally get clear readings. It’s a reminder that numbers don’t always capture the reliability needed on the bench or in scale-up.
For Phenazine-2,3-diamine, the stakes are high. This compound finds use in dye formulations, electrochemical systems, and, at times, as an intermediate for more complex molecules. Impurities wreck color performance, kill battery longevity, or poison reaction pathways. Data shows that research teams settling for lower-grade batches often chase their tails with inconsistent results. Time wasted, materials lost, conclusions in doubt.
Reputable suppliers run their batches through fine-toothed combs. Analytical tools like gas chromatography and NMR spectrometry can spot contaminants right down to fractions of a percent. A top-quality batch means fewer headaches, whether you’re setting up a new test, scaling up a process, or just hoping to get repeatable results. Often, a company offering 99%+ pure Phenazine-2,3-diamine will back it up with certificates showing not just purity, but also the profile of trace contaminants.
Chemists and process engineers have run into problems with poor records, substitutions by brokers, and spotty handling, which knock quality down before it reaches your hands. My own work has suffered from untracked storage time and poor handling—especially when someone thought a zip-lock bag counted as “secure.” Moisture and oxygen get into an unprotected batch and end up changing the color and reactivity overnight.
No one needs to accept inconsistent quality. Sourcing material from companies with a strong record matters. Ask for method sheets and third-party analyses, not just a sticker on the bottle. I always store sensitive compounds in tightly sealed vessels with low humidity. Make sure whoever is ordering knows their stuff—one quality slip-up sets research back or risks final product safety.
Partnership with trusted suppliers matters as much as any technical skill in the lab. Choosing price over quality brings hidden costs: reruns, failed upscaling, regulatory headaches. My suggestion is to focus on traceable supply, solid documentation, and reliable support—not just the catalog number. For Phenazine-2,3-diamine, that commitment pays off in reliable outcomes, fewer headaches, and confidence in every step from bench to industrial process.
People who work in research know that tall chemical cabinets and well-labeled bottles are just basics. The real skill kicks in with compounds that have a quirky side. Phenazine-2,3-diamine lands in that group. It doesn’t offer much grace if you ignore its moods. A little too much heat in the room, some stray moisture on the shelf, and that bottle may turn into a headache—not always in the literal sense, but sometimes even that. The physical characteristics and stability of this compound demand respect from anyone handling it.
It makes me think of a chemistry lab from my college days. We kept a bottle of Phenazine-2,3-diamine on the bottom shelf, partly in fear and partly by habit. That spot was never reached by sunlight, which is ideal. Sunlight and this compound pair poorly. The UV rays can slowly break things down at the molecular level. The result? Decomposition products you don’t want drifting through your air or contaminating your results. No one enjoys tracing ruined data back to a lazy storage choice. Luckily, an amber glass bottle closes off this threat. The thick tinted walls block out most light and with a tight cap, leaks stay at bay.
The average rule holds: aim for below room temperature, somewhere between 2 to 8 degrees Celsius. People treat this as dogma with a reason. Cooler air slows the pace of reactions. In my experience, the main lab refrigerator does the job, but never let the bottle sweat. Every time you move things in and out, temperature swings tempt condensation into the bottle. Water droplets don’t play well with Phenazine-2,3-diamine, often sparking off reactions you did not ask for. Once or twice I witnessed the telltale color shift that warns you things are heading south. If you spot this, don’t just tighten the lid. Find a safe way to dispose and replace the stock.
Storing this compound away from oxidizers and acids matters more than a printed sign on the door. Mixing chemical families can end the lab day early—or much worse. Once, a neighboring shelf held a forgotten bottle of organic peroxide, and the air in the room seemed to get “thicker” over the weeks. Nobody pin-pointed a disaster in the making until an inspection saved the day. Staying organized with proper separation, sturdy containers, and impeccably clean utensils has clear payoffs. I always say, don’t trust a scoop that’s been in an unknown jar down the bench.
Regular checks make a difference: tight seals, dry exteriors, clean labeling with both chemical and hazard information. I keep a log of bottle-open dates, which helps spot subtle changes or breakdown. Don’t skip simple steps like gloves and goggles, even when just moving bottles around. Real safety comes from boring routines that turn into habits. Strong ventilation in the storage area minimizes the risk if something slips past your caution. I learned to prefer slightly over-cautious practices after seeing close calls up close.
Safe storage of Phenazine-2,3-diamine boils down to smart, disciplined routines. Controlled cold, a sealed and shaded container, and thoughtful segregation of chemicals are not luxury—they are baseline for safe and reliable research. Responsible handling protects both people and the work they’re building each day.
Phenazine-2,3-diamine rarely makes the front page, but anyone who steps near a lab bench soon learns how tricky it behaves. This stuff comes from the phenazine family, and a few cousins in that group show up in dyes, pharmaceuticals, and even cancer research. But laboratory work sometimes means trading curiosity for care, and that's true here too.
Exposure stories usually come down to inhalation, skin contact, or accidents in a crowded workspace. Even at a distance, fine powder can float, and anything that turns into dust gets into places you’d never expect. If you’ve worked with powdered chemicals, you likely remember nose and throat irritation the minute you lifted a slightly suspect lid.
Phenazine-2,3-diamine usually irritates, and sometimes it burns. Red, itching hands, or watery eyes mark sloppy work. Enough exposure can go beyond that—chronic contact sometimes attacks your skin, and some studies say certain phenazines might have mutagenic tendencies, meaning that long-term, reckless handling opens a door to worse health problems.
In the lab, stuffing chemicals anywhere saves no one time. Dedicated cabinets with clear hazard labels catch my eye, especially when the alternative has bottles jumbled like puzzle pieces. Keep phenazine-2,3-diamine containers tightly closed. Use containers made of materials able to resist chemical action and keep them dry. Dampness triggers breakdown and odd reactions, and the resulting mess always follows you home on your clothing or shoes.
Cotton clothes and bare hands have no place here. I remember one stubborn undergraduate who shrugged off gloves, only to spend the afternoon at campus health with a rash. Gloves, eye protection, and a fitted lab coat block the worst. Respirators—preferably with particulate filters—matter when you need to weigh out or manipulate phenazine-2,3-diamine powders. Anyone who skipped masks during a dusty synthesis knows the soreness that follows.
Fume hoods play a starring role in all chemical handling, and this one’s no exception. Those shields, even if foggy or lined with notes, separate the worker from an invisible cloud of risk. Eating and drinking near chemicals still happens, mostly when people get lazy or miss a lunch break, but it never ends well. Washing up before leaving the lab means the ride home stays just another drive, not an emergency.
Someone always ends up cleaning a spill. For phenazine-2,3-diamine, dry brushing sends more dust into the air, so damp cloths and HEPA vacuums work better. Absorbent pads, nitrile gloves, and a calm head keep the job simple. Eyes or skin splashed by accident call for a long rinse, right away, and medical help. Sticking stubbornly to written protocols saved me from more near misses than I'd like to count.
Training saves more lives than any fancy equipment. People ignore the basics until the basics have the last word. Hazard assessments, regular reviews, and respect for the chemicals—especially stubborn, unpredictable ones like phenazine-2,3-diamine—build a safer space for everyone on the team.
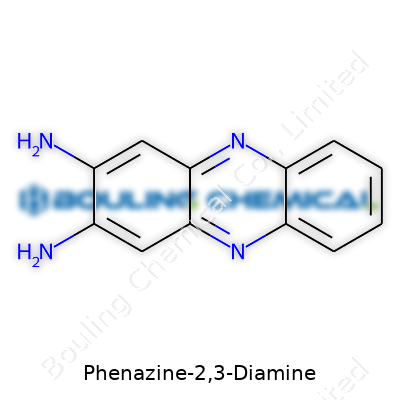
| Names | |
| Preferred IUPAC name | 2,3-Diaminophenazine |
| Other names |
2,3-Diaminophenazine C.I. 50005 Diamino-2,3-phenazine |
| Pronunciation | /fəˈnæz.iːn tuː θriː daɪˈæm.iːn/ |
| Identifiers | |
| CAS Number | 2378-86-1 |
| Beilstein Reference | 147697 |
| ChEBI | CHEBI:82924 |
| ChEMBL | CHEMBL2342064 |
| ChemSpider | 22170884 |
| DrugBank | DB08485 |
| ECHA InfoCard | 12e606e9-3d3a-4ee1-8421-8500fb2b805a |
| EC Number | EC 1.10.3.10 |
| Gmelin Reference | 93072 |
| KEGG | C06586 |
| MeSH | D010612 |
| PubChem CID | 13555 |
| RTECS number | SS9625000 |
| UNII | D9H06K571U |
| UN number | 2811 |
| CompTox Dashboard (EPA) | DTXSID7020376 |
| Properties | |
| Chemical formula | C12H12N4 |
| Molar mass | 196.23 g/mol |
| Appearance | Red to brown powder |
| Odor | odorless |
| Density | 1.305 g/cm3 |
| Solubility in water | Insoluble |
| log P | 0.3 |
| Vapor pressure | 1.81E-4 mmHg at 25°C |
| Acidity (pKa) | 3.83 |
| Basicity (pKb) | The basicity (pKb) of Phenazine-2,3-diamine is **5.0**. |
| Magnetic susceptibility (χ) | -0.55×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.768 |
| Dipole moment | 3.53 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 193.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -193.8 kJ/mol |
| Pharmacology | |
| ATC code | D03BB13 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation |
| GHS labelling | GHS02, GHS07, GHS08 |
| Pictograms | GHS06,GHS08 |
| Signal word | Danger |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| Flash point | Flash point: 221.4°C |
| Autoignition temperature | 615°C |
| Lethal dose or concentration | LD50 (oral, rat): 615 mg/kg |
| LD50 (median dose) | LD50 (median dose): 480 mg/kg (rat, oral) |
| PEL (Permissible) | Not established |
| REL (Recommended) | 100 µg/m³ |
| Related compounds | |
| Related compounds |
1,2-Diaminobenzene 2,3-Diaminopyridine Phenazine Phenazine-1-carboxylic acid Phenazine methosulfate |