Science rarely follows a straight line. Phenazine-1,2-diamine started as a byproduct on the bench of 19th-century organic labs, known to those who studied dyes and coal tar derivatives far before modern medicine caught on. Researchers who first separated phenazine compounds often worked with crude tools, filtering dark, pungent mixtures and testing colors on fabric. The curiosity driving those early chemists, who often lacked formal safety gear, built the backbone of synthetic organic chemistry. Over decades, the story moved from trial-and-error dye applications into fine-tuned molecular design, following the real needs of growing fields like pharmaceuticals and microbiology. As the world began looking for answers to infections and new routes for technical applications, scientists took another look at phenazine derivatives, including phenazine-1,2-diamine, searching for more than just bright color.
Phenazine-1,2-diamine comes as a yellow to brown crystalline solid, a staple in some chemical inventories for decades. As a staple intermediate, it forms a bridge between crude synthesis and specialized end-products. Chemists prize its stability and the wide range of transforms possible on the molecular ring. Sometimes, the most helpful raw material doesn’t grab headlines but quietly makes possible everything from advanced pigments to some forms of anti-tubercular agents.
On the bench, phenazine-1,2-diamine feels dense to the touch, with a melting point in the moderate range, around 140-150°C. In my memory, the scent stands mild but distinct, setting it apart from other phenazine compounds with sharper odors. The molecule, C12H10N4, packs two amine groups at the 1 and 2 positions on the heterocyclic ring. This matters much for solubility, which leans toward organic solvents such as ethanol and dimethyl sulfoxide, but less so for water. Heating can oxidize the compound, and exposure to strong acids or bases changes its reactivity. From the standpoint of storage, containers should stay sealed and in the dark, since air and moisture slowly degrade quality.
Purchasing phenazine-1,2-diamine means checking for the basic attributes: purity beyond 98%, precise batch numbers, and clear labeling, usually with respected identifiers like CAS No. 92-54-6. There’s little tolerance for uncertain suppliers or vague labeling in this sector. Standard packaging uses amber glass to lessen the impact of light and oxidation. Safety sheets get checked, as mishandling causes skin and respiratory irritation. I always made sure that my stockroom tagged the hazard codes and expiration date clearly, because old or contaminated stock can put a project—or a person—at risk.
The most established preparation kicks off with phenazine itself, which undergoes a nitration step, followed by reduction under controlled, cool conditions. Reduction commonly employs tin(II) chloride in hydrochloric acid or catalytic hydrogenation, methods that depend on the scale and purity demands of a given project. Large-scale syntheses fall back on batch reactors with careful monitoring, since incomplete reactions or contaminants easily ruin product quality. In the lab, hands-on chemists remember this as a process demanding patience with temperature and filtration, especially during work-up and washing.
Researchers don’t treat phenazine-1,2-diamine as just a static final product. Modification through alkylation of the amine groups, acylation, and oxidation broadens its field significantly. In hands-on work, it often reacts with electrophiles to build more complex molecules, and scientists discover new routes for derivatives every year. For me, this simple-looking ring was always a launchpad—one where a small tweak could create a brand-new pigment or push further into antimicrobial activity. Oxidative coupling reactions, sometimes using catalysis or mild oxidants, bring a whole array of colors and bioactive agents into reach.
Outside formal chemical circles, few recognize “phenazine-1,2-diamine”. It goes by several other names: O-diaminophenazine to some, 1,2-diaminophenazine to others. The key is knowing that product catalogs, regulatory filings, or scientific publications don’t always speak the same language. Inaccurate use of synonyms causes real world complications that reach from shipping mishaps to regulatory violations. Consistency matters at every step, from the order form to the waste disposal log.
Direct experience has shown me: safety protocols mean understanding more than PPE slogans. Phenazine-1,2-diamine needs gloves, goggles, and proper ventilation, as fine dust irritates lungs and skin. Spills, though rare, call for careful sweeping and a full clean-up. Labs and pilot plants using this compound keep up-to-date MSDS on hand, use chemical-resistant surfaces, and train up new staff on good handling habits. In the case of accidental contact, water and soap get used as the first line of response, but more severe exposure still needs professional medical follow-up. Waste streams containing phenazine-1,2-diamine require specific disposal, often by incineration or chemical degradation, instead of dumping in standard waste streams.
Phenazine-1,2-diamine lives many lives. Pigment technology was one of the first big applications, with the compound serving as a precursor to stable, bold dyes for textiles and inks. Pharmaceutical research found new paths: researchers discovered how certain derivatives show antibacterial and anti-tubercular properties that challenged even resilient strains of bacteria. Analytical chemistry relies on its redox reactivity—an example would be sensors that change color thanks to the phenazine core. Specialty coatings, catalysts in cross-coupling reactions, and even early batteries used phenazine-based molecules, and that speaks to its range. This compound slides easily from basic science into technology, changing only the minds and methods of the chemists handling it.
The best labs don’t let old molecules gather dust. Research on phenazine-1,2-diamine covers everything from mechanism studies—how electrons move through the ring—to new transformations that bring out overlooked reactivity. Drug discovery sticks close to this scaffold. Academic groups, as well as pharma giants, screen phenazine-based compounds for antibiotics at a time when resistance rises. On the technical side, researchers push for eco-friendly syntheses, aiming for less waste and safer reagents. Partnerships between industry and universities feed off this curiosity, racing to patent new derivatives or find a better route. Progress marches quickly, but sharing methods and negative results keeps the work honest.
Toxicity gets tested before any real application reaches people or the environment. Phenazine-1,2-diamine shows moderate toxicity in lab animal studies, especially at high doses. Chronic exposure damages organs and disrupts cellular systems, so regulatory bodies like the EPA and EU REACH list strict exposure limits. I remember one project halting for months after finding trace phenazine metabolites in unexpected waste streams. That experience cemented my respect for rigorous workplace monitoring and transparent reporting. Researchers also track biodegradation, as some forms linger in soil or water and disrupt local systems if not controlled. Routine bloodwork and air monitoring protect workers in manufacturing, while both environmental chemists and toxicologists still study long-term risks.
Future outlooks sit close to practical needs. The growth of drug-resistant microbes means phenazine-1,2-diamine and its kin keep showing up in research pipelines as starting points for new antibiotics or cancer treatments. R&D groups also keep digging into its electrochemical flexibility, wondering if old molecules can power new batteries or supercapacitors. Pigment technology moves toward greener, more stable colors, a place where phenazine derivatives offer new options. Regulations and better industrial practices shape future production, putting pressure on manufacturers to refine methods and minimize waste. Open data-sharing and smarter regulatory frameworks promise better safety and more rapid discovery—because each use of phenazine-1,2-diamine demands as much responsibility as curiosity.
Phenazine-1,2-diamine isn’t a chemical folks stumble across in daily life, but it plays a unique role in research and industry. I’ve come across it in the context of microbiology research, where scientists look for compounds that can nudge bacteria into acting differently. This molecule often turns up in studies that focus on bacterial communication and defense systems.
One of the corners where phenazine-1,2-diamine gets attention is in the lab, as a useful building block for researchers. Scientists use it to make dyes, especially those that end up in analytical testing. It helps produce vibrant colors that signal the presence of certain substances in a test tube. For years, chemists have cared about how easily this compound can accept or donate electrons. That trick fits the world of sensors and detection kits, where signaling seems to rely on tiny shifts in color or light. Anyone who’s peered at chemical reactions on a benchtop recognizes the practical side of having indicators that can reveal changes without expensive machines.
Across medicine, phenazines get a close look for their effects on microbes. Some bacteria in nature naturally churn out phenazine-style molecules to compete with other bugs in the soil. Phenazine-1,2-diamine hits the radar because it shares traits with antibiotics—think about attacking the cell walls of certain bacteria or getting mixed up in the chemical signaling that helps bacteria clump together and form stubborn infections.
There’s a long process before anything makes it from a lab experiment to a pharmacy shelf. Toxicity, stability, and how it interacts with living cells—they all need answers. Still, for young researchers hunting for new approaches to infection, these sorts of small molecules offer a spark. Turning one of these leads into a useful treatment takes collaboration between chemists, doctors, and manufacturers.
One issue in science circles with phenazine-1,2-diamine is safety. Most chemicals with the power to change living cells don’t always do so gently. Researchers keep gloves on and ventilation strong. People dealing with it in bulk, like those making dyes, face rules to track exposure, avoid spills, and prevent problems for workers and waterways.
The hunt for cleaner chemistry pushes for greener alternatives. I often see research groups aiming to tweak the molecule itself or design processes with fewer leftovers. Sometimes, swapping solvents or working at room temperature helps. That kind of small practical shift can cut costs, protect workers, and keep the planet a little safer for the next person down the line.
Cutting-edge research keeps prodding scientists to use phenazine-1,2-diamine more thoughtfully. Partnerships between public and private sectors matter. Universities want to stay creative, but scale and safety matter most in real-world use. The chemical industry always faces a balancing act: make something that works, keep it safe, and watch the waste stream.
For folks outside the chemical or research world, the story here is about the compounds working behind the scenes. They support technologies in healthcare, diagnostics, and beyond, showing how much depends on strong, creative problem-solving. Keeping an eye on health and the environment makes a difference, not just in labs but where these chemicals end up after the fact.
Phenazine-1,2-diamine calls itself C12H10N4 on the chemical registry. Put simply, you get twelve carbon atoms, ten hydrogens, and four nitrogens making up the molecule. What’s interesting about this formula? It’s balanced enough to create a decent ring system, which can coax some unusual and valuable chemical behaviors.
Picture a pair of six-membered benzene rings joined in a back-to-back configuration, sharing two carbon atoms. That fused ring skeleton builds the base known as “phenazine.” For Phenazine-1,2-diamine, toss in two amine groups (-NH2), anchoring them to the first and second positions of the skeleton. Where exactly? Right next to each other, hanging on to one edge of the fused rings. It's as if the molecule grew earmuffs.
A little fun comes into play with fused aromatic rings. These compounds don’t just look pretty under a microscope. Their chemical layout sets up electron sharing, passing charge along the ring path, almost like copper wire. Add those -NH2 groups, and the molecule starts to behave in new ways. It grabs protons or passes electrons, crucial for redox reactions. In the world of dyes, this is especially useful — these molecules can shift or give off color with only a nudge in pH or a swap of electrons.
There’s more to phenazine-1,2-diamine than academic curiosity. Chemists and microbiologists keep coming back to it. I’ve seen it in action for its antimicrobial properties. The molecule’s ring system seems to slip into places where bacteria don’t want strangers. Researchers have found it promising in the fight against certain plant diseases, which matters for food security. Antibiotic resistance nips at the heels of modern medicine, so every chemical lead feels worth a look.
Like most aromatic amines, phenazine-1,2-diamine comes with a warning label. Early on in my lab days, we kept the stuff in a tightly sealed amber bottle, and for good reason. Compounds like this can irritate skin and eyes. There have been reports of mutagenic effects from some similar structures. The message is clear: don’t let curiosity turn into carelessness.
The buzz about phenazines in pharmaceuticals, agriculture, and materials science keeps growing. Synthetic chemists might tinker with its backbone, shifting functional groups or extending the ring system to adjust the molecule’s properties. As someone who’s had to run TLC plates and NMRs on dozens of these compounds, my take is the real progress will come from targeted drug development and careful biological testing. Skipping steps on safety checks can wreck an entire research pipeline.
Instead of chasing every new derivative, labs can team up with computational chemists. Modeling how these molecules interact with proteins or cell membranes cuts down pointless trial and error. Digital simulations demand less raw material and help pick out the best contenders before feeding them to a petri dish. We’ve saved months in the past using these tools, keeping the search for new antibiotics smart and lean.
Phenazine-1,2-diamine, with its compact formula and distinctive structure, has a track record of attracting researchers. Every detail — from ring fusion to the amine attachments — shapes its place in chemistry and biology. As interest in new treatments and crop protection grows, it seems clear we’ll see it pop up in new contexts. Staying mindful of its strengths and its risks keeps the field moving forward.
Storing chemicals often sounds simple until you find yourself dealing with the likes of phenazine-1,2-diamine. This compound, known in some labs as o-phenylenediamine, has a reputation: handle with care or face the consequences. Working with chemicals like this has shaped how I think about lab safety. I’ve seen gloves degrade, labels fade, and storage containers fail. Every mistake offers a reminder: not all chemicals accept shortcuts.
Phenazine-1,2-diamine’s tendency to oxidize and form harmful byproducts places it in a different league compared to table salt or even aspirin. Keeping it away from light, moisture, and air becomes more than a formality—it’s a matter of health. In my experience, careless storage turns a stable compound into a hazard ready to spill, corrode, or ignite. Direct contact might irritate your skin, and inhaling dust means trouble for your lungs. Each bottle deserves clear labeling, a tightly sealed cap, and a home far from sunlit windows or heat sources.
Most laboratory protocols point to refrigeration, and that lines up with best practices. Store the compound at low temperatures, but not in the typical freezer by tomorrow’s lunch. I still remember a co-worker’s panicked face after mixing up chemical vials with leftovers, and nobody wants a repeat of that. Use dedicated, chemical-safe refrigerators. Put desiccants on the shelf, and trust in tight packaging—preferably amber glass to block stray light from seeping in. Label everything with both the date received and the date opened, because degradation sneaks up faster than expected.
Expired compounds don’t belong on the shelf, no matter how tight the research budget looks. Phenazine-1,2-diamine changes over time, and ignoring expiration dates can introduce mystery variables into your work. If a spill does happen, my policy calls for a dust mask, gloves, and dedicated cleanup absorbents—not just a quick swipe with a paper towel. Ventilation matters. Chemical fumes can linger, so working under a fume hood lowers the risk. Even the most cleaned-up surface deserves a second wipe with detergent and water.
So many lab mishaps trace back to storage and labeling. With phenazine-1,2-diamine, color changes in the bottle hint at trouble. If the compound shifts from yellow or brown to something darker, it signals breakdown. Dispose of it through the right hazardous waste channel—never down the drain. For disposal and storage alike, always use containers rated for hazardous substances. Keep logs of storage locations and quantities. It helps during inventory and adds accountability if something does go wrong.
Poor storage raises the odds of fire, chemical burns, and ruined experiments. Taking time upfront to check labels, sort by hazard class, and document everything pays off not just in safety but in better science. Instituting checklists or regular audits in the lab might sound tedious, but it catches problems before chemicals turn against their users. People can get complacent, myself included. Yet stories of accidental exposure or fires—shared in hushed tones at lab meetings—keep everyone aware that careful storage isn’t just a rule. It’s a necessity that protects both people and projects.
I’ve handled my share of odd chemicals over the years—inside labs, on factory floors, and in garden sheds where labels faded to nothing. When Phenazine-1,2-Diamine comes up, it pays to pay close attention. This chemical—an aromatic diamine—turns up in selective dyes, research settings, and specialized diagnostics. It may sound obscure, but in chemical safety, obscurity doesn’t always mean harmless. Several laboratory manuals and occupational safety sheets spell out its health risks. Skin, eye, and respiratory irritation often top the list for people who have direct contact. Stronger warnings raise concerns over potential toxicity if someone ingests it, or gets fine airborne particles in their lungs.
Coming face to face with lab agents, most experienced techs operate in a culture of caution. I remember watching a veteran chemist put on respirators for a quick weighing session—more for certain compounds than others. Substances like Phenazine-1,2-Diamine land among those that demand real care. Animal studies link similar compounds with DNA damage or mutation risk, though research on this molecule itself is far from complete. Still, the possibility of genotoxic or carcinogenic effects shouldn’t be brushed aside. For this reason, strict handling guidelines kick in before opening a single ampule: chemical-resistant gloves, eye protection, fume hoods. People in some industries try to engineer solutions so fewer workers ever get exposed. Sometimes, the best practice is swapping these agents out for something safer when the chemistry allows.
I once worked at a site near a creek that ran bright yellow after a rainstorm—a memory that makes pollution feel personal. Few people worry about synthetic chemicals drifting into the water table until the fish float belly up. Phenazine-based molecules break down slowly outdoors. This makes them a risk not just for workers, but for anyone downstream. Studies show that compounds with nitrogen-heavy backbones have a knack for sticking around and moving through soil. It’s worth noting the spillover—literally. Plant run-off or improper storage can send traces of these chemicals into public spaces, especially when robust containment plans get skipped in a rush.
Real improvement never comes from assuming that the old way will do. The most practical step involves better training. Too often, folks in laboratories or manufacturing settings cut corners, especially under pressure. Regular training sessions and reminders about which chemicals call for full PPE help keep people from paying the price for speed. Transparency about risks creates a workplace where folks speak up if something looks off. Industry rarely wants to take the hit of new safety equipment or substitute reagents, but ignoring the costs of exposures ends up worse for everyone. Funding research into safer dyes or looking for green chemistry options nudges the field forward, but it takes time and a willingness to change long-set habits.
Not every chemical compound becomes a boogeyman overnight. Trust in science means following the trails that research reveals and owning up to risk when it’s documented. Phenazine-1,2-Diamine doesn’t appear in every household, but it has real power to cause harm where it's used. In my own work, the lesson sticks—read the label, heed the warnings, and make safety ordinary. Genuine change means more than writing rules: it calls for respect for experience, chance for new voices to demand better, and the courage to swap dangerous habits for safer ones.
Step into any chemistry storeroom and you’ll see dozens of bottle sizes jostling for space. It’s no different with Phenazine-1,2-diamine. The most common options I’ve run into? Small glass bottles—usually 5 grams, 10 grams, or 25 grams—dotting the shelves in labs built for research. These tiny amounts let chemists test reactions without sinking budgets on wasted chemicals. Researchers gravitate toward these because minimal risk comes from opening a small bottle multiple times; it keeps contamination at bay and avoids moisture sneaking in.
Go up in scale, and you’ll find plastic or glass jars—anything from 50 grams to 250 grams. Mid-sized orders hit the sweet spot for folks running repeated syntheses over weeks or juggling several grad students on one big project. You start finding these sizes in teaching labs and specialty chemical suppliers who know some folks work just above “hobby” level. Anything bigger than 250 grams? Here’s where industry or pilot plants step in.
Industrial outfits don’t mess around with dinky vials. They often need Phenazine-1,2-diamine by the kilo or more. Five-kilogram, ten-kilogram, and even 25-kilogram fiber drums show up in chemical supply catalogs. I once toured a dye manufacturing line; workers rolled out hefty drums—sturdy plastic, topped tight, with every bit of powder sealed against the air. These drum sizes slash costs per gram, cater to manufacturing that eats through pounds a week, and reduce the hassle of frequent reorders.
There’s nothing glamorous about big buckets of chemical powder, but there’s practicality at play. Large producers order in bulk to cut down paperwork and shipping costs, and to be honest, nothing compares to the peace of mind when supplies don’t run out mid-batch.
Not every supplier stocks every size. Some users want special anti-static containers or liners to suit certain climates. Because Phenazine-1,2-diamine can stain skin and clothes deep yellow, containers arrive labeled with warnings, and sometimes double-wrapped for added safety. Custom batch sizes turn up—for example, a university lab once split a shared order into 50-gram jars for professors who didn’t trust communal scoops.
One real concern: safe handling. Many containers include moisture-absorbing packets, and nobody with lab experience trusts a bulk drum without a tamper-evident seal. Ask anyone who’s watched a cleanup after a spill—proper packaging comes from hard lessons.
Modern labs feel the pressure of waste reduction, too. Small bottles mean lots of glass recycling, but they cut down on product spoilage. Drums save on packaging but could leave kilos unused if shelf life lapses or projects shift focus midstream. Some suppliers are switching to recycled plastics or offer returnable containers—one step in the right direction, especially when every scrap of hazardous waste needs documenting and safe disposal.
In the end, nobody wants to pay for more than needed or throw money away through waste. Size selection in chemical packaging stays firmly practical: it comes down to quantity, safety, and what fits a lab's or a factory’s real day-to-day usage. Each size from micro-bottle to industrial drum reflects needs born in real-world use, lessons learned the hard way, and—just maybe—a few warehouse managers who’d rather stack fewer boxes.
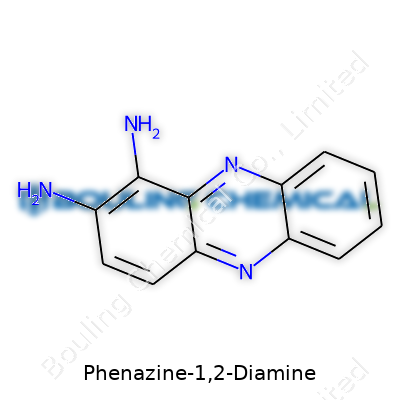
| Names | |
| Preferred IUPAC name | 5,10-Diaminophenazine |
| Other names |
o-Phenylenediamine OPD 1,2-Diaminobenzene 1,2-Benzenediamine |
| Pronunciation | /ˈfiː.nəˌziːn waɪ.tuː daɪˈæˌmiːn/ |
| Identifiers | |
| CAS Number | 92-54-6 |
| Beilstein Reference | 58202 |
| ChEBI | CHEBI:28498 |
| ChEMBL | CHEMBL2111576 |
| ChemSpider | 52998 |
| DrugBank | DB16060 |
| ECHA InfoCard | 03aac205-ef4d-426e-bb05-11247ca91da4 |
| EC Number | 205-503-2 |
| Gmelin Reference | 85937 |
| KEGG | C06584 |
| MeSH | D010610 |
| PubChem CID | 13525 |
| RTECS number | SS7525000 |
| UNII | AYC1H7G185 |
| UN number | UN1673 |
| CompTox Dashboard (EPA) | urn:text:DTXSID9044368 |
| Properties | |
| Chemical formula | C12H12N4 |
| Molar mass | 196.24 g/mol |
| Appearance | Red to brown powder |
| Odor | amine-like |
| Density | 1.22 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 0.2 |
| Vapor pressure | 5.14E-4 mmHg at 25 °C |
| Acidity (pKa) | 13.32 |
| Basicity (pKb) | 7.54 |
| Magnetic susceptibility (χ) | -0.7×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.751 |
| Viscosity | Viscous liquid |
| Dipole moment | 1.51 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 223.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -9.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2041 kJ/mol |
| Pharmacology | |
| ATC code | D08AX01 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS05|GHS06 |
| Pictograms | GHS07 |
| Signal word | Danger |
| Hazard statements | H302 + H312 + H332: Harmful if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | Precautionary statements for Phenazine-1,2-Diamine: "P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 100°C |
| Autoignition temperature | 540 °C |
| Lethal dose or concentration | LD50 (oral, rat): 480 mg/kg |
| LD50 (median dose) | 640 mg/kg (rat, oral) |
| NIOSH | SS64750 |
| PEL (Permissible) | PEL (Permissible) of Phenazine-1,2-Diamine: Not established |
| REL (Recommended) | 0.1 mg/m3 |
| Related compounds | |
| Related compounds |
1,2-Phenylenediamine Phenazine |