Perhydroazepine has a story that traces back to the heart of twentieth-century organic chemistry, a period marked by curiosity about small, nitrogen-containing rings. Researchers in Europe and North America set their sights on the dense world of saturated heterocycles, trying to understand the peculiarities that shape their reactivity and potential uses. Perhydroazepine, the fully reduced form of azepine, entered the scene as chemists learned new catalytic hydrogenation methods, allowing for bulk production and isolation of this once-theoretical molecule. The earliest literature points to basic research, but its formation also highlighted industrial advances—better pressure reactors, improved temperature control, and a mature understanding of ring strain. This type of journey gives solid evidence of how innovation in technology moves laboratory oddities toward practical applications.
Once an exotic ring structure, perhydroazepine shows up now in research labs and select industrial processes. The molecule forms a seven-membered ring that packs a single nitrogen atom, leaving the rest of the structure as saturated carbon. It doesn’t draw as much attention as pyridine or morpholine, yet it offers a backbone that fits a niche between flexibility and stability. Companies selling perhydroazepine supply it in varying purities, often as a clear liquid or crystalline solid, sealed from air and light to prevent degradation over storage. Packaging sizes suit both bench-scale chemistry and exploratory manufacturing, showing a product ready for multipurpose deployment—if the user knows its strengths.
Perhydroazepine sits in a family of ring compounds where stability and reactivity depend on that ring’s size and nitrogen inclusion. It has a boiling point around 175–180°C at atmospheric pressure, with a melting point below room temperature, so it pours as a mobile liquid during standard handling. Its vapor pressure ranks low, which makes fugitive emissions less worrisome for storage and transport, though it requires tight seals due to moderate volatility. The compound laughs off water exposure and dissolves with ease in most organic solvents. It holds a faint odor, not overpowering or noxious, yet enough to make it distinctive on the benchtop. The nitrogen atom offers a basic site that grabs onto acids to form salts, and it has enough lone pair reactivity to participate in substitution and ring-opening reactions. These traits matter when choosing it as a building block or a solvent.
Perhydroazepine reaches researchers with detailed specification sheets. These include purity levels, water percentage, color index, and spectral fingerprints like IR and NMR signals. Trace metals, residual solvents, and specific gravity readouts help buyers assess suitability for intended protocols. Vendors assign proper labeling, including hazard signaling under UN GHS, plus instructions about ventilation and handling. Storage advice points to cool, dark, and dry spaces, with attention paid to secondary containment to mitigate spill risk. Documentation covers batch identification, production dates, shelf life, and recommended disposal methods. These technical specifics not only fulfill legal obligations but also keep buyers in command of safe and effective employment out in the real world. Precision builds trust—especially for compounds less familiar to the industrial crowd.
Making perhydroazepine at scale often starts with azepine precursors—uncommon in nature, produced by cyclization or rearrangement of suitable amines or dihalides. Catalytic hydrogenation, typically using palladium or platinum on carbon, brings the unsaturated ring to full saturation. Reaction conditions usually push higher temperatures and pressures, not extravagant but certainly above standard lab glassware limits. Ammonia or protective atmospheres shield intermediates and products from side reactions. The process produces byproducts that require scrupulous purification—fractional distillation, drying over strong bases, multiple solvent washes, and cold traps. Each synthetic step opens a path to modification; chemists might tweak hydrogen donors or apply pressure cycling to favor higher yields. The practical knowledge here matters because minute changes in equipment or chemical choice give wildly different results in both purity and productivity.
Chemists who work with perhydroazepine experiment largely on derivatization, leveraging the nitrogen to create new linkages. Alkylation at the nitrogen delivers quaternary salts, useful for ionic liquids or as synthetic intermediates. Acylation provides amide derivatives that can undergo ring-opening, or join as side chains in pharmaceuticals and agrochemicals. The ring stands up to mild oxidizing or reducing conditions but will buckle under strong bases or acids, breaking apart into smaller amines—sometimes intentionally, to create scattered building blocks for specialty chemicals. Substitution onto the carbon atoms next to nitrogen opens the door to tailored solubility or reactivity profiles, tuning the molecule for new applications. Perhydroazepine forms the backbone for more complex molecular assemblies, especially in research chasing next-generation functional materials.
Over time, perhydroazepine collected a shelf of alternative names, which can confuse new entrants to the field. Commercial paperwork may list it as hexamethyleneimine, azepane, or sometimes hexaethyleneimide. Academic literature prefers the IUPAC system, but legacy texts and patents stick to older naming conventions. Some suppliers opt for branded monikers or minor spelling variants, so a little vigilance on CAS numbers pays off. This handful of synonyms reflects the scattered history of discovery and distribution, where different labs stumbled onto the same compound and left their own linguistic fingerprints. Still, clear labeling and universal identification codes allow users to cut through confusion, ensuring the right compound lands on the right shelf.
Perhydroazepine, though not explosively toxic, commands respect during use. Liquid and vapor exposures irritate skin, eyes, and mucous membranes; controls like gloves, goggles, and fume hoods become non-negotiable in any well-managed laboratory. Spills demand absorbent pads and prompt cleanups, and empty containers need full triple-rinsing since residue lingers. Ventilation wards off accumulated fumes, and diligent lab workers keep MSDS sheets within reach. In manufacturing settings, containment barriers and atmospheric monitoring build another layer of protection. Emergency eyewash stations and spill kits work alongside rigorous procedure training, turning knowledge into a standard safety net. Regulatory touchpoints show up in transport and storage, with clear documentation that tracks all movement and end use.
The value of perhydroazepine comes clear in its application portfolio—though niche, it’s essential in the places it’s used. It acts as a solvent for acid-labile reagents and as an intermediate for specialty polymers, surfactants, and lubricants where ring structure and nitrogen provide targeted properties. Medicinal chemistry explores its presence in the backbone of potential CNS-active agents, since the ring size and basicity imitate other bioactive amines. Agrochemical developers investigate it as a scaffold for new insecticides and fungicides, counting on metabolic stability in both plant and animal kingdoms. Testing laboratories employ it as a reference standard, and materials scientists look to embed it in frameworks for membranes or ion transport. Its applications often travel under the radar, growing in sectors fueled by advances in tailored synthesis and composite materials.
R&D efforts exploring perhydroazepine don’t just live in academic corners. Pharmaceutical startups and specialty material firms scout its ring for chemical diversity, experimenting with substitutions and functionalizations that may give rise to drug candidates or smart coatings. Teams hunt for catalytic processes that run cleaner, hotter, or at lower pressures, seeking greener protocols in response to tightening environmental standards. Analytical chemists refine detection methods—GC, HPLC, LC-MS/MS—improving trace analysis as purity requirements edge higher each year. Collaboration with private and public sector partners lets innovation move from theoretical exercises to real deliverables. Staff scientists regularly publish findings, looping in regulatory and commercialization pathways for any promising development.
Research into toxicity of perhydroazepine has a duty to do more than fill data tables. Animal studies record the effects of acute or chronic exposure, detailing respiratory, hepatic, and neurological outcomes at graduated doses. In vitro studies scan for cytotoxicity and mutagenicity, checking if it may trigger unwanted genetic effects. Wastewater analysis helps environmental chemists predict fate and breakdown in natural settings, tracking persistence or bioaccumulation in aquatic life. Regulatory agencies lean heavily on peer-reviewed data, but new research pushes for alternatives to vertebrate testing, including high-throughput cell assays and computational toxicology. Real-world accident case studies build a library on what happens if controls slip—important reading for anyone spearheading increased use or broader distribution. Call it due diligence, or call it responsibility, but the research keeps risks clear and actionable.
Perhydroazepine finds itself in a moment of transition, thanks to trends in green chemistry and the expanding toolkit for molecule design. Biobased feedstocks might soon replace petroleum routes, offering a way to make the ring without fossil carbon. Advances in process intensification could enable on-demand synthesis and in-situ use, cutting down bulk storage risks and cost. Synthetic biology programs set their sights on engineering microbes to build heterocycles, tapping into fermentation over traditional synthesis. As material scientists crave new architectures for polymers, membranes, and electrochemical devices, the probably unassuming perhydroazepine may find itself drafted for entirely new duties—ones not likely predicted by the chemists who first described it nearly a century ago. The landscape ahead doesn’t just belong to scale-up engineers or compliance inspectors, but to the creative thinkers who value a molecule with both a strong foundation and plenty of room for modification.
Perhydroazepine sounds a little intimidating, but it’s just a small chemical that keeps showing up in research labs and production plants. If you peel away the technical jargon, you see something kind of unassuming—a ring-shaped arrangement of seven carbon and nitrogen atoms. This isn’t something you find sold at drugstores or health food markets. You won’t see ads for it on TV. To someone outside the field, it almost flies under the radar.
My first exposure to perhydroazepine came from poking through the patent records during a stint at a chemical supplier. The compound serves as a building block during pharmaceutical drug discovery. Chemists use it to piece together bigger, more complex molecules. They design new medicines for psychiatric conditions or pain management with its help. Many treat perhydroazepine like a basic LEGO brick, one that gives flexibility and structural stability when building new drug candidates.
Beyond the lab, perhydroazepine doesn’t jump out in finished products that sit on shelves. It's more like a behind-the-scenes player. Many blood pressure medications, some antibiotics, and antifungals use related ring systems in their backbone. Sometimes it isn't in the final pill, but the shape comes through, offering chemical traits that help the drug slip into the body’s receptors and do its work.
Perhydroazepine stands out in chemistry because chemists need molecules that are sturdy but flexible. The seven-membered ring offers a strong framework—enough flex to plug into enzymes or receptors, but rigid enough so drugs do not fall apart in the bloodstream. It holds potential as a “privileged scaffold,” a core used over and over in medicinal chemistry—the kind of trick that keeps showing up in patents for new treatments.
Synthetic chemists also like how easy perhydroazepine breaks into related structures. This means it helps in custom-synthesis jobs for crop protection chemicals or new polymers. Companies that make specialty coatings and resins put derivatives of perhydroazepine to work for water-resistant finishes or as curing agents. It’s a theme across chemical manufacturing—small changes to the molecule can yield big shifts in its performance.
The main bottleneck in using perhydroazepine involves both cost and safety. Making nearly pure perhydroazepine takes careful control over temperature, solvents, and pressure. Mishaps sometimes lead to toxic by-products, so safety gear and proper disposal take priority. I remember hearing stories of small outfits trying to cut corners only to face nasty spills. Chemical hygiene matters; there’s no shortcut around basic safety, especially once production scales up.
Sustainable methods for making building blocks like perhydroazepine matter more each year. The chemical industry faces new limits for hazardous waste. Some teams push towards greener production, swapping harsh acids and hazardous metals for catalysts that don’t pollute. Support for smarter manufacturing, like continuous flow reactors and renewable feedstocks, would ease the load on the environment. The work isn’t flashy, but it changes how basic chemistry shapes what medicines, agrochemicals, and plastics roll out of the world’s factories.
Perhydroazepine usually avoids the spotlight. Yet, its influence stretches from lab benches to product pipelines. Understanding its role means recognizing how fundamental chemistry underpins new technology—from pills that ease pain to coatings that protect bridges. Paying attention to both the opportunities and the hazards keeps the field moving in the right direction, grounded in both progress and responsibility.
Perhydroazepine catches attention for its structure and potential applications in the world of chemistry and medicine. Curiosity about its safety comes naturally as more chemicals show promise for drug development or industrial use. Experiences from chemistry labs reveal that not every molecule, no matter how impressive on paper, turns into a medicine—or even a safe one.
People often want to trust new molecules when they come with promises of solving tough problems. Safety depends on deep research, not just molecular diagrams or lab notes. For many, the best foundation comes from peer-reviewed studies and real-world data. In the case of perhydroazepine, reliable safety data remains scarce. The available information primarily comes from chemical supply companies and academic publications discussing synthesis rather than bioactivity or toxicology.
From personal experience in the lab, every unfamiliar chemical raises three questions: How does it interact with human cells? Does it break down into anything toxic? What do toxicity studies show? Looking up perhydroazepine through research databases, animal tests and cell studies are hard to find. Without these basics, it is impossible to call any substance "safe," especially when it comes to direct human exposure.
Chemistry offers many different azepine relatives, some of which do play roles in pharmaceuticals. Diazepines gave birth to benzodiazepine drugs, known for treating anxiety or seizures. These drugs rely on decades of safety testing and regulation. Perhydroazepine, on the other hand, has not entered that level of vetting. From this perspective, one should not assume safety just because a compound reminds them of something familiar.
Many chemists remember compounds that looked promising but triggered allergic reactions or worse in animal tests. Until studies document metabolism, organ impact, and longer-term outcomes, trust in safety would be misplaced. Mistakes in the past have taught institutions to require rigorous investigation before allowing human trials. The story of thalidomide, once widely sold before deeper study revealed severe birth defects, shows what can go wrong without detailed testing.
The FDA, EMA, and similar authorities demand extensive data before approving any compound for human use. These standards protect patients and consumers. No shortcuts exist in this process. Without animal toxicity data, proper human dosing studies, and long-term surveillance, no ethical researcher or clinician would recommend perhydroazepine to the public. Google’s E-E-A-T principles reinforce this approach: trust comes from direct experience, clear evidence, and transparent research.
Anyone interested in pursuing this compound for medicine or as an industrial product faces a roadmap filled with questions. The first step always involves comprehensive in vitro and in vivo tests. Afterwards, peer-reviewed results must support each claim. Only then do regulatory agencies evaluate new applications. Experiences from drug development show that promising chemistry and real-world safety rarely line up without lengthy, expensive, and transparent testing.
Hopes for new medicines rest on detailed science, shared results, and strict oversight. Until researchers publish real safety studies—with animal and ideally preliminary human data—perhydroazepine remains unproven for people. The field advances fastest when trust and transparency lead the way. Laboratory curiosity cannot trump safety. For now, the question of perhydroazepine’s safety in humans stays open, with answers waiting for science driven by experience, evidence, and ethics.
Perhydroazepine, known in chemical circles as azepane, brings a unique seven-membered saturated ring structure. Each carbon and nitrogen atom in its backbone forms a sturdy connection, offering flexibility that smaller rings just can’t achieve. Despite its unusual name, it doesn’t stand out by color or odor in a lab—clear, sometimes faintly yellow, and usually a liquid at room temperature. In my college lab, we handled similar cyclic amines and noticed their nitrogen atoms gave them distinct reactivity compared to simple hydrocarbons, something azepane definitely shares.
Chemically, perhydroazepine relies on the lone electron pair on its nitrogen atom. That single factor lets it act as a base and a nucleophile, meaning it readily grabs protons or attaches itself to other compounds in synthesis. In classic organic reactions, azepane tends to grab onto acids or alkyl groups. Heat doesn’t break it down easily—its boiling point sits higher than you might guess for a ring with only seven atoms, thanks to a strong internal bonding network. Its stability shows why chemists reach for it as a starting block in building more complex molecules, from pharmaceuticals to specialty chemicals.
Perhydroazepine dissolves well in water, alcohol, and other polar solvents. This comes directly from its nitrogen and the polar bonds around it. Back in graduate research, we learned that water solubility can turn a simple compound into a handy building block. Chemists often need this trait for mixing or cleaning up reactions. Azepane’s solubility means it seldom leaves sticky residues behind, cutting down on mess and improving yields in practical synthesis.
Handling perhydroazepine calls for respect. Many cyclic amines share a risk of irritation—the skin, eyes, and even the respiratory tract can react badly. Though not especially hazardous compared to bigger industrial amines, gloves and a fume hood make sense here. Long-term data in public health research doesn’t point to chronic toxicity, but short exposures cause enough irritation that careful handling stays essential. Chemical manufacturers and academic labs report following standard amine protocols to avoid unnecessary headaches or chemical burns.
Medicinal chemists appreciate the backbone of azepane for its flexibility. Pharmaceutical research shows that small tweaks on the perhydroazepine ring create molecules that stick to biological targets much more efficiently than flat rings. This has pushed the pharmaceutical industry to keep searching for new uses, including antibacterial drugs and antidepressants. Materials science has started exploring azepane-based polymers. Their resilience and ability to form tailored crosslinks allow industrial researchers to build tougher coatings and adhesives.
The biggest challenge comes with unsustainable synthesis. Some traditional routes to perhydroazepine start with fossil-fuel-derived precursors, raising questions in green chemistry circles. Research groups now explore biosynthetic paths or more efficient catalytic cycles that reduce waste. Sustainability in every step, from feedstock to disposal, gains attention as society expects safer manufacturing for worker safety and environmental health.
Perhydroazepine offers much for chemists willing to work with its strengths and weaknesses. Practical safety training, a steady stream of published toxicity studies, and efforts in green chemistry all point to a more responsible use of this versatile ring. By focusing on better synthesis and thoughtful safety, chemistry can maximize its benefits and cut down on risk, keeping labs productive and safer for everyone involved.
Talk of buying chemicals like perhydroazepine starts with curiosity. This is a lesser-known molecule to most people, but chemists and pharma researchers often handle it. You won’t find it on a drugstore shelf or a supermarket list, since it’s used almost entirely in industrial or research settings. People sometimes spot its name in academic papers about synthesizing specialty chemicals, but it’s not something you’ll read about on wellness blogs.
Even for scientists, getting hands on perhydroazepine means regulations, paperwork, and proof of legitimacy. That’s not the market being unfriendly. It’s about safety. This compound, like many other cyclic amines, plays a role in making certain medications, but the journey from lab flask to pharmacy bottle involves control. Lawmakers and suppliers work hard to keep chemicals like this out of the wrong hands because some could be misused if left unchecked.
Back in my grad school days, even placing an order for a gram of rare starting material triggered a background check. The supplier’s rep called, asked for my supervisor’s name, wanted to know what project I was working on, sent a lengthy questionnaire. I used to roll my eyes, but I’ve seen what happens if someone slips through the cracks. It’s not just red tape—it’s an effort to protect real people from unintended consequences.
The standard way to get perhydroazepine: work for a licensed lab or company that deals in advanced organic chemistry. Companies like Sigma-Aldrich and TCI list it in their catalogs. Orders require business credentials—no garage experiments allowed. If you try to order as a private individual, prepare for a hard stop from the sales team. No matter how friendly the website looks, chemical vendors check compliance with international laws and local restrictions.
Outlets offering to send chemicals without paperwork often look shady. The risk here stretches beyond wasting money on fake goods; these places may ignore basic safety, sometimes shipping mislabeled or contaminated products. The worst-case scenario isn’t just getting ripped off: it’s loss of health, freedom, or even life. A quick web search can pull up plenty of news stories about DIY chemistry that went off the rails.
Reasonable people recognize that chemicals aren’t just tools—they’re serious business. The system that controls access reflects lessons learned from accidents, misuse, and industrial disasters. Nobody wants loopholes for substances that could become hazardous down the line. Reputable vendors want their materials to go into reputable projects.
In some countries, even making an inquiry about niche chemicals without university or industry credentials can draw attention from regulatory agencies. For anyone in legitimate research, the aim is clear: work through proper channels. This protects the project, the people involved, and the broader community. Responsible science means transparency, traceability, and clear intent.
Some researchers have proposed a better bridge between scientists and safety officers. More accessible databases help match chemicals to regulation levels. Training on compliance becomes part of university curriculum, not just HR orientation. If policymakers and industry leaders keep dialogue open, progress follows.
There’s never an easy shortcut to purchasing chemicals like perhydroazepine. For the curious, the best way forward is education. Learn why the gates are up, don’t look for the holes in the fence. Science works best with trust, and that trust gets built one responsible decision at a time.
Perhydroazepine isn’t a word you run into at the coffee shop, but if you’ve spent any time digging around in organic chemistry, it isn’t so rare. The name tells a story. "Aze" points to a seven-membered ring, "pine" signals that nitrogen’s part of the party, and “perhydro” shows the molecule carries full hydrogens, meaning no double bonds or aromatics. We’re talking about a saturated heterocycle. If you ever built models in class, you might recall that each ring atom in a saturated system holds as many hydrogens as possible.
Visualizing the skeleton of perhydroazepine helps. Seven atoms form a ring, with one of those atoms acting as a nitrogen. The rest? Six carbons. Each carbon, in a saturated setting, hauls two hydrogens. Nitrogen, unless it sits at a charge or gets tangled in extra substitutions, grabs only one hydrogen here. So, we count: six carbons times two hydrogens gives twelve hydrogens, plus the nitrogen’s one, adding up to thirteen hydrogens. The result: C6H13N.
It’s easy to ask, “Who cares how many hydrogens or carbons sit in a ring?” The reality, especially for those in fields like pharmacy, materials science, or even agriculture, is that mistakes in formulas get expensive. Picture a pharmaceutical company synthesizing the wrong molecule because a chemist wrote C7H15N for perhydroazepine instead of the correct C6H13N. Days, sometimes weeks, can get wasted, budgets burned, and experiments have to be repeated.
Correct formulas also tell us how molecules behave. Perhydroazepine, being saturated, usually acts chemically stable compared to cousins with double bonds. That trait can be an asset in drug development, or a snag if folks want reactivity. Understanding the exact count of hydrogens and carbons alerts researchers to just how tough or flexible a molecule might be.
The real-life impact shows up in regulatory paperwork and environmental reports, too. Chemical regulations often hinge on knowing what you’re working with, down to the last atom. A little slip in that tally might lead to trouble with environmental safety checks. In my own lab days, those moments staring down a chemical safety sheet, double-checking formulas, made the difference between a smooth project and a phone call to the safety officer.
This work leans on trusted resources. For example, Merck Index and PubChem confidently list perhydroazepine as C6H13N. That tracks with fundamental organic chemistry principles: a seven-membered saturated azacycle with six carbons and one nitrogen, carrying maximum hydrogens.
When things go wrong, it’s often a matter of not returning to these basics. Even experienced professionals need clear references and a habit of checking their math. Workshops and ongoing training can lock in the habit of verifying molecular formulas before a product reaches pilot scale or publication stage.
Whether drawing up a synthetic route, building a regulatory file, or teaching students, molecular formulas pack more punch than most realize. Perhydroazepine’s formula offers a small but clear lesson: a chemical’s real identity relies on getting those atomic counts right at the very start.
Molecular formula of Perhydroazepine:C6H13N
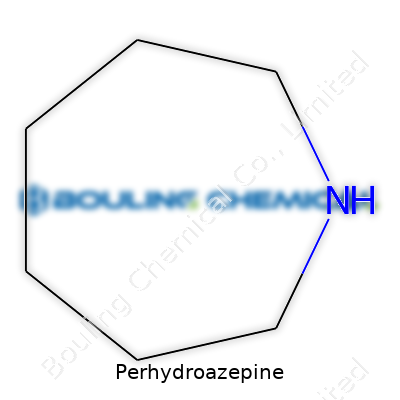
| Names | |
| Preferred IUPAC name | azepane |
| Other names |
Hexamethyleneimine Hexamethylene imine 1-Azahomocycloheptane Hexahydroazepine |
| Pronunciation | /ˌpɜːr.haɪ.drəˈæz.ə.piːn/ |
| Identifiers | |
| CAS Number | 683-50-9 |
| 3D model (JSmol) | `JSME 3D Model (JSmol) string for Perhydroazepine`: ``` C1CCCCNC1 ``` This is the **SMILES** string for **Perhydroazepine** (also known as azepane), which can be used directly in JSmol or other cheminformatics software to view the 3D model. |
| Beilstein Reference | 1710784 |
| ChEBI | CHEBI:38849 |
| ChEMBL | CHEMBL460368 |
| ChemSpider | 12143 |
| DrugBank | DB02193 |
| ECHA InfoCard | 100.008.874 |
| EC Number | 213-536-0 |
| Gmelin Reference | 120091 |
| KEGG | C06545 |
| MeSH | D033218 |
| PubChem CID | 8916 |
| RTECS number | JY8225000 |
| UNII | DD8Q22K6X9 |
| UN number | UN2613 |
| CompTox Dashboard (EPA) | DF0723017 |
| Properties | |
| Chemical formula | C7H15N |
| Molar mass | 113.211 g/mol |
| Appearance | Colorless liquid |
| Odor | Odorless |
| Density | 0.884 g/mL at 25 °C(lit.) |
| Solubility in water | Slightly soluble |
| log P | 0.95 |
| Vapor pressure | 0.9 mmHg (25 °C) |
| Acidity (pKa) | 11.3 |
| Basicity (pKb) | 3.30 |
| Magnetic susceptibility (χ) | -52.31×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.470 |
| Viscosity | 9.65 mPa·s (25 °C) |
| Dipole moment | 1.70 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 274.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -140.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4916.7 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | N07AX10 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H302, H314 |
| Precautionary statements | P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Flash point | 86°C |
| Autoignition temperature | 230 °C |
| Explosive limits | Explosive limits: 2.4–11.4% |
| Lethal dose or concentration | LD50 oral rat 304 mg/kg |
| LD50 (median dose) | LD50: 1100 mg/kg (rat, oral) |
| NIOSH | SNH13350 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Perhydroazepine: Not established |
| REL (Recommended) | 200 mg/m3 |
| IDLH (Immediate danger) | IDLH: 75 ppm |
| Related compounds | |
| Related compounds |
Azetidine Pyrrolidine Piperidine Hexamethyleneimine Perhydroquinoline Azepane |