Digging into the story of nicotine sulphate opens a chapter on the intersection of chemistry and agriculture. Centuries ago, farmers and scientists looked for ways to protect their crops, especially before the flood of synthetic solutions. Tobacco, an ever-present crop, already carried a reputation for its natural insect-repelling traits. Early extraction methods set the groundwork for isolating nicotine in more concentrated and usable forms. This moved quickly from folk wisdom to industrial-scale processes as global trade flourished. Old scientific reports capture how researchers tapped into sulfuric acid as a carrier, transforming unstable nicotine into nicotine sulphate — not just for ease of transport, but as an answer to growing pest issues. In the 20th century, factories across Europe, America, and Asia produced this compound to meet the booming needs of commercial agriculture. Over time, safety concerns and stricter regulations checked its widespread use, but its mark remains on both the history of plant protection and the evolution of chemical safety standards.
Nicotine sulphate typically shows up in liquid form, packed in metal drums or high-strength plastic containers. This compound carries a potent punch. As far as insecticidal products made from natural resources go, nicotine sulphate has stood out because it bridges the gap between pure plant extracts and fully synthetic chemicals. Labels on these products usually flag high toxicity, which cannot be overstated. You’re not just dealing with a generic chemical solution. Nicotine sulphate offers rapid knockdown of soft-bodied insects and a handful of persistent pests. Although its market presence has fallen off due to regulations and the rise of safer options, pockets of agricultural practice still use it for specific crops and situations where quick action matters.
What sets nicotine sulphate apart is its blend of properties — oily, water-miscible, and deeply pungent, with a pale yellow to amber color. Its molecular formula, C10H14N2·H2SO4, reveals a structure designed for potency. Solubility in water reaches high levels, which helps with making spray solutions on site. Under moderate conditions, it remains stable, although strong bases or prolonged exposure to light eventually break it down, releasing its sharp, unmistakable odor. On the technical spectrum, it offers low vapor pressure but, if left exposed, slowly evaporates and can linger in the air. Anyone opening a container straight from storage will immediately notice its bitter, acrid smell, which often triggers warnings that go beyond just label symbols.
Producing and selling nicotine sulphate comes with strict rules, from chemical purity to child-resistant caps and pictogram-rich warning labels. Most commercial grades hover around 40% concentration for agricultural use, with clear details about the presence of stabilizers and allowable impurities. Labeling guidance borrows from global harmonization systems, flagging acute toxicity, environmental hazards, and first-aid steps. Regulatory agencies demand batch-level traceability, expiration dates, and distinct hazard statements. In my experience, older warehouses sometimes store nicotine sulphate in faded drums — a risk for any operation, since recent legislation in countries like the United States and European states bans its unsupervised sale or use. Modern product sheets stress that even small spills pose real hazards, from skin absorption to environmental contamination.
The pathway from leaf to sulphate starts with tobacco extraction. This means soaking cured and shredded tobacco in water and an acidic medium. The solution pulls out the alkaloid, which then reacts with sulfuric acid in controlled steps. What comes out is a salt, not the base, so it resists easy evaporation and accidental ignition. Filtering and further purification rounds remove plant debris and residual oils. Commercial-scale production uses closed-loop systems to protect workers and the environment. Anyone working with the raw extracts notices an oily residue impossible to ignore. Efficient processing demands rigorous equipment cleaning, strict temperature control, and ventilation at every stage.
Nicotine sulphate comes from a simple acid-base reaction, but researchers and chemical engineers have explored different tweaks. Small changes in concentration, temperature, or reactant ratios alter crystal size and solubility. Over the decades, scientists have tried to convert the sulphate back into base nicotine for more direct applications or even to neutral compounds for detoxification. Sometimes this involves using alkaline solutions, which chemically liberate the original alkaloid. Efforts to reduce its environmental toxicity have focused on breaking it down with oxidizers or friendly microbes, which turn the molecule into less harmful fragments. That work continues as labs try to find cleanup methods after accidental release — not just for nicotine sulphate but for similar organics in fertilizers and pesticides.
The commercial world uses a handful of names for nicotine sulphate, such as "nicotine hydrogen sulfate", "nicotine sulfate", or simply "tobacco extract sulfate". Some older catalogs reference "Black Leaf 40" or “Nicotine Insecticide” — reminders of its time as a go-to bug killer. In laboratory circles, the compound shows up as "nicotinium sulfate", matching systematic naming conventions. Trade names often reference either the percentage of nicotine, such as "NicSul 40", or hint at its intended markets, including horticulture and specialty plant protection.
Nicotine sulphate sits at the top tier for occupational safety risks. Direct skin contact, inhalation, or accidental ingestion can quickly produce toxic symptoms, from dizziness to respiratory failure. Standard operating procedures require gloves, chemical splash goggles, and full-coverage clothing. Many modern facilities use closed handling frames, negative-pressure rooms, and real-time gas monitoring just to avoid worker exposure. As regulations grew stricter, businesses needed to offer safety training, ready access to wash stations, and regular health monitoring for those in jobs touching nicotine compounds. Disposal brings another set of challenges. The Environmental Protection Agency and similar authorities treat waste nicotine sulphate as hazardous. Each country sets unique thresholds and incineration guidelines, but no one escapes the paperwork or stewardship duties.
Even as synthetic chemistry expanded, nicotine sulphate has held a spot, mostly for legacy crops and biologically sensitive farms. Small-scale farmers sometimes look for natural-based treatments as alternatives to organophosphates and neonicotinoids. Apple orchards, specialty vegetables, and even some greenhouse hobbyists apply it for stubborn aphid infestations — typically as a foliar spray after dilution. Despite its strength, pest resistance rarely builds, thanks to the compound’s complex mode of action. Organic certification almost never allows it anymore, but some transition farms find it helpful during conversion periods. Outside farming, a few niche industries consider nicotine sulphate for research into biological controls or as a reference compound to judge newer, targeted insecticides.
In the lab, nicotine sulphate serves as much more than a throwback pesticide. Research groups in toxicology, pharmacology, and environmental sciences study its breakdown, uptake in plants, and interactions with soil microbes. College chemistry departments might run experiments testing natural product extraction methods or reaction efficiencies. These projects often yield insights on how other alkaloid-based agrochemicals behave. Technology has pushed some teams to build slow-release formulations, seeking better target coverage with less risk of ground runoff. Real-world use informs academic work, which in turn shapes future regulations and best practices. Several companies and agricultural researchers keep nicotine sulphate in their toolkit for testing biopesticide delivery systems and remediation strategies after accidental overspray.
Few substances match the toxicity profile of nicotine sulphate for its size and ease of absorption. Toxicity studies across species show it moves rapidly through biological membranes and bloodstream. This high absorption brings quick onset of symptoms, so safety studies focus on minimum lethal doses, chronic exposure, and synergistic effects with other household chemicals. Labs publishing on environmental impact found both negative and nuanced outcomes — harm to beneficial insects stands out, but breakdown rates and roadside runoff patterns suggest mitigation options. Animal testing, increasingly under ethical review, underscores that repeated exposure raises serious risks not just for acute poisoning but long-term neurological impact. Even today, regulatory agencies lean on decades-old studies as they weigh whether more modern, targeted alternatives can handle the same jobs with less harm to bystanders and wildlife.
Looking forward, nicotine sulphate’s place becomes more selective. Increased consumer demand for transparency and stringent residue limits narrow its market role. Most commercial growers adopted safer chemicals or biological solutions, and the class of products built around nicotine faces declining regulatory support. Still, the molecule offers a template for green chemistry advocates seeking safer, plant-based alternatives. Research investment trickles steadily into better delivery methods, non-toxic breakdown pathways, and recycling ideas for spent materials. Some agricultural engineers and environmental chemists see nicotine sulphate as a learning tool instead of just an end product — a window into how to innovate without repeating past pollution problems. The challenge lies in balancing effective pest management with genuine commitment to environmental health.
Nicotine sulphate sits on the shelf as a colorless to pale yellow liquid, but its history and function go deep, especially in the world of agriculture. For nearly a hundred years, farmers have trusted this compound to protect crops. And for good reason—this substance works as a powerful insecticide, taking down aphids, thrips, leafhoppers, and a whole list of other pests. Long before the big chemical companies pushed out synthetic pesticides, growers turned to extracts from tobacco, with nicotine sulphate leading the way.
Picture tomato fields or rows of cabbage back in the 1940s. Synthetic options hadn’t taken over yet. Farmers would mix nicotine sulphate with water and sometimes soap, using it as a spray to keep bugs away from prized vegetables. It offered quick knockdown, driving away pests without hanging around on produce for too long.
I remember my grandfather mixing up a batch in a big barrel behind the barn, his hands yellow from nicotine-stained leaves, swearing by its results. It always amazed me how he managed the risks, knowing enough to keep the solution away from animals and family. Stories from that time remind us what close contact with powerful natural chemicals looks like—and why safety always counts.
Instead of wide-sweeping poisons, nicotine sulphate attacks the nervous system of insects. It paralyzes them fast, which means little time for plant damage. After the work gets done, the compound breaks down under sunlight and rain, lowering residue left on food, an important concern before tough food safety rules. Still, that hardly means it’s harmless. This stuff isn’t just rough on bugs; people and animals can get poisoned easily through skin, lungs, or mouth. The CDC, World Health Organization, and other health experts all warn about its toxicity. In the hands of someone careless, this solution can kill.
Look at accident reports, and you’ll spot the reason regulators in many places phased out nicotine sulphate. Modern-day farmworkers still land in hospitals if they cross paths with this chemical without enough gear or training. Symptoms—nausea, headaches, breathing trouble—point to nerves reacting to just a little dose.
We’ve got other choices now. Safer synthetic insecticides and improved integrated pest management practices help keep both workers and the environment safer. In many countries, using nicotine sulphate faces heavy limits or outright bans—not because it stops fighting bugs well, but because the risks hang over people, animals, and water. On top of that, overuse brought on resistant bugs, a trend seen with any chemical used again and again.
Still, talk to old-timers and you’ll hear a certain respect for this liquid. They knew its power better than anyone, respecting both what it fixed and what it threatened. Lessons from the era of nicotine sulphate stick around: Always respect what you handle, know the risks, and never assume something “natural” won’t carry a punch. Crops feed us, but the means of keeping them healthy should never come at the cost of health and safety. Growers, regulators, and scientists share responsibility here—learning from history, so fewer people pay a price for protection.
Nicotine sulphate comes from tobacco plants. It’s often called an organic pesticide, though that label doesn’t make it safe. Farmers used this chemical to kill bugs long before many modern pesticides appeared. There’s no questioning its power against insects. The real concern comes from what nicotine does beyond its immediate target.
Spraying nicotine sulphate puts both farm workers and consumers at risk. The body absorbs nicotine through skin, lungs, or mouth. Exposure on the farm can easily cause symptoms like nausea, headaches, weakness, or even collapse. Some workers have landed in hospitals after getting the stuff on their hands or breathing it in. As someone who’s worked on a small farm, I’ve heard plenty about “tobacco poisoning”—it’s not rare, and it knocks you flat.
Some people think washing crops solves the problem. But nicotine can stick around on leaves, fruits, and vegetables. Even after washing, residues sometimes linger. A few old government studies showed nicotine traces on tomatoes and greens, even after harvest. The chemical breaks down over time, but picking too soon puts that risk directly on the dinner table.
Nicotine sulphate kills more than just crop pests. Bees, butterflies, and other pollinators run into trouble if they contact sprayed plants. Once pollinators go, the health of an entire farming system breaks down. Birds that feed on poisoned bugs feel the impact, too. This isn’t theory—researchers have found nicotine residues in bee hives and bird nests near sprayed fields.
In the United States, nicotine-based pesticides have mostly vanished from store shelves. The Environmental Protection Agency lists nicotine as a restricted-use pesticide. Australia and Canada also limit it sharply. Governments see enough risk to human health and the environment to justify these strong measures. Even inspectors testing imported produce look out for this residue.
Only a handful of places still allow it—and that usually comes from either outdated rules or weak enforcement. Farmers using nicotine sulphate face growing pressure from exporters, local regulators, and even neighbors who worry about water and soil contamination.
Safer choices exist. Some farmers have turned to neem oil, pyrethrin sprays, or simple mechanical pest barriers instead. Integrated pest management lets growers use bugs and birds as natural control, cutting chemical sprays. Over time, most growers who switched over saw fewer headaches—literally and figuratively.
Cost plays a big part in the decision. Modern pesticides can seem expensive, but they usually pack smaller health risks when used right. Training and protective gear help, too. I remember switching from “natural” tobacco concoctions to newer products on my own family’s plot—it felt like a relief to worry less about my skin or lungs every morning.
Trying to balance pest control and safety pulls in more voices lately—consumers, health professionals, and farmers alike. A healthy farm community grows from shared responsibility and honest look at risks. Nicotine sulphate worked for a time in farming history. Science moved on, and so should we. Smarter choices give rural families, farm workers, and eaters better odds for well-being without gambling on an old chemical that’s proven its danger.
Nicotine sulphate doesn’t ask for much to be dangerous. It’s toxic enough that only a few drops can cause severe harm or death, even through skin contact. This isn’t just a chemical that needs a locked cabinet and a warning sign. Every story of exposure in a poorly ventilated shed or accidental contact from a leaky bottle says one thing loud: direct handling without regard for safety leads to tragedy.
A lot of people forget that proper storage isn’t only about locking it away. I grew up around farms, and someone always had pesticide bottles tucked away under a sink or left in a pickup truck. Convenience like that is tempting—and it's exactly what goes wrong. Nicotine sulphate belongs in a cool, dry space, far from sunlight, heat sources, or open flames. That's not only about reducing chances of fire or spoilage. It’s about lowering the risk someone finds it by accident, especially if that someone is a child or a farmworker unaware of the danger.
Plastic bottles crack, metal rusts, and glass might shatter on a rough day. Factory-sealed containers do their job for a reason. They don’t leak fumes or drip out liquid on your hands. If someone ever needs to transfer nicotine sulphate, it should be done with care—in a space with good ventilation and away from anyone else. Every spill matters. The toxic vapors don’t give a warning before they do harm.
Labels fade. Caps get swapped. There’s always the urge to pour into a smaller bottle for “easy measuring.” That’s not convenience; it’s an accident waiting to happen. Keeping the original packaging means anyone touching that bottle knows exactly what’s inside. Even a chart or a log listing dates and quantities goes a long way. It sounds simple, but sharp labels save lives, especially if emergency responders ever show up.
I’ve watched neighbors use pesticides in sheds with only a small window. The air inside gets thick and your nose picks up a sharp, chemical bite. That’s danger, not just discomfort. Storage spaces and mixing areas need ventilation—not a cracked door, but a fan or an exhaust. Airflow means that fumes won’t settle and make someone sick or worse.
National and local laws don’t only exist to complicate paperwork. They reflect real cases where exposure went wrong. Keeping nicotine sulphate secure doesn’t only protect people working with it. Fines, lawsuits, and public health scares follow when people ignore safety rules. The legal side serves as another reminder that this substance isn’t just hazardous—it’s deadly when managed carelessly.
Gloves, goggles, and long sleeves don’t make anyone invincible, but they’re the last line of defense. Anyone who’s ever tried to scrub a spill from their skin remembers the fear and the burn. Accidental absorption happens fast. People sometimes think a careful pour is enough, or that gloves “just slow things down.” Stories of people getting sick after a single contact prove that the right gear is always worth the extra minute.
Even with storage protocols and PPE on hand, the real safeguard comes from experience and alertness. Regular training reinforces respect for the dangers at hand. It also keeps people sharp about avoiding shortcuts. Routine checks, quick refreshers before handling, and a culture that calls out risky behavior do more than any rulebook ever could.
Nicotine sulphate stands among the most toxic materials used in gardening and agriculture. Many people don’t realize just how dangerous it gets, especially in its concentrated form. Even skin contact or inhalation of vapors can trigger serious reactions—nausea, rapid heartbeat, dizziness, spasms, or sudden weakness. Consuming a small amount, even by accident, can send someone to the hospital in minutes. It’s classified as a Hazard Class I substance for a reason.
Folks in pest control or greenhouse work see warnings and thick gloves as routine, but accidents often stem from cutting corners or skipping gear in a rush. My grandfather ran a small orchard and swore by the stuff for controlling aphids—he also warned us to keep our distance until he’d showered and changed. He saw too many friends get sick by breathing in fumes or splashing the liquid onto bare arms.
Using nicotine sulphate safely doesn’t start at the mixing station, it starts by setting up with intention. Work outside or in an air-ventilated shed. Anyone handling the material covers up: chemical-resistant gloves, a long-sleeved shirt, pants that reach the boots, and goggles. I’ve seen younger workers brush this off on hot days, reasoning that “just a splash” won’t keep them down. That attitude sends people to the ER.
Masks matter too. If you do not seal out vapors, all those warnings—brain fog, vomiting, and even seizures—become real risks. Basic cloth masks fail. A respirator with organic vapor cartridges does a better job, especially during mixing or spraying.
Always measure with dedicated tools. If a measuring cup’s been near nicotine sulphate, keep it locked away from kitchen use. Use a clean, labeled container, and mix only enough for the job. Leftovers shouldn’t get dumped down the drain. They can end up in waterways, poisoning fish, birds, or even pets in the neighborhood.
Before spraying, double-check the equipment for leaks. Sprayers drip, and a few drops will soak pants, socks, or shoes. Spray in a pattern that avoids wind-blowback. If the breeze shifts, pause and move. Even people gardening nearby risk harm—always give a loud heads-up and rope off the area if it’s a public or shared space.
After finishing, the gear needs a full clean with soap and water. Hands get scrubbed—no quick rinse, but a thorough wash under running water. Dirty clothes shouldn’t go into the regular laundry load; wash them separately or wear disposable coveralls. Store all bottles and mixing tools in a locked cabinet. Kids are always curious, especially when bright labels or bottle shapes catch their eyes.
If nicotine sulphate gets on the skin, full removal comes before anything else. Strip contaminated clothing and wash with plenty of water. Inhaling too much vapor or swallowing the liquid sends up alarms—get medical help fast. Time lost fiddling with remedies adds to the danger.
Many regions restrict or outright ban nicotine sulphate now, in favor of safer pesticides or integrated pest management. Biological controls—a swarm of ladybugs or parasitic wasps—give results in home gardens without putting people or pets at risk. Replacing nicotine sulphate with these options helps prevent accidental poisoning, both for workers and for the next generation growing up on family farmland.
Gardeners and farmers know that pest outbreaks can make or break a season. Nicotine sulphate used to provide a quick knockdown for sucking insects like aphids, thrips, and mites. Many old-timers remember their parents dusting tobacco juice over greens, swearing by results that seemed to speak for themselves. Today, people reach for commercial solutions, but the basics haven't changed: the amount you mix spells the difference between an effective spray and a hazard.
The classic recommendation for nicotine sulphate usually comes up as a 0.05% to 0.15% solution in water for field spraying. That translates as roughly 30 to 60 milliliters of a 40% nicotine sulphate concentrate in 10 liters of water. Commercial advice—like older guides for vegetable plots—sometimes leans toward a higher end of that range for tough infestations, especially where mites or scale insects dig in. Some citrus farmers report better results with the higher rates, but caution is front and center due to human toxicity concerns.
Nicotine doesn’t know the difference between a pest and a person. Just a skin splash or mist settling in the lungs can cause headaches, nausea, dizziness, and much worse if the dose climbs higher. Field operators have shared stories of falling ill after spraying in windless, humid mornings. Even the USDA flagged nicotine as too dangerous for home use, which explains why it dropped out of favor among many gardeners and farmers. Organic farms, in particular, rarely rely on it now. The low safety margin for people, pets, and pollinators gives cause for second thoughts.
Nicotine sulphate hasn't disappeared entirely. In some regions where access to modern pesticides is limited, people still count on it during outbreaks of mealybugs or heavy aphid loads. Instructions matter: wear gloves, full sleeves, and avoid breathing the mist. Never use it near harvest, since the compound lingers on produce and can poison whoever eats it. European Union regulators banned it outright, while some countries restrict sales to trained professionals. In my own work with small-scale growers, I suggest checking for updated local regulations before considering a nicotine sulphate treatment. Fact is, regulatory loopholes can lead to accidental misuse.
Safer options often do the job without the risk. Neem oil and insecticidal soaps bring fewer worries about food safety. Biocontrol with ladybugs, lacewings, or fungal pathogens knocks back aphids and mites without leaving dangerous residues. Careful crop monitoring and rotating pest management help, too. Whenever people ask about nicotine sulphate, I point them to local extension services or university-backed guides for the most up-to-date and region-appropriate advice.
The bottom line for anyone considering nicotine sulphate: measure accurately, respect the warnings, and know there are usually better routes available.
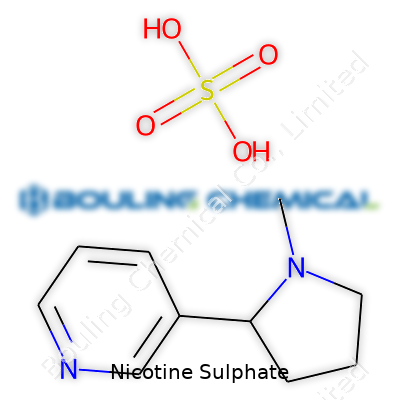
| Names | |
| Preferred IUPAC name | bis(3-(1-methylpyrrolidin-2-yl)pyridine) sulfate |
| Other names |
Black leaf 40 Nicotine sulfate solution Nicotine sulphate insecticide Nicotine-40 Nicotine sulphate 40% Tobacco extract |
| Pronunciation | /naɪˈkəʊtiːn ˈsʌlfeɪt/ |
| Identifiers | |
| CAS Number | 65-30-5 |
| Beilstein Reference | 1718735 |
| ChEBI | CHEBI:38595 |
| ChEMBL | CHEMBL2106659 |
| ChemSpider | 21516 |
| DrugBank | DB11446 |
| ECHA InfoCard | 100.030.317 |
| EC Number | EC 200-214-2 |
| Gmelin Reference | 8376 |
| KEGG | C05329 |
| MeSH | D009538 |
| PubChem CID | 5960 |
| RTECS number | WN6650000 |
| UNII | 6M3C89ZY6R |
| UN number | UN1654 |
| Properties | |
| Chemical formula | (C10H14N2)2·H2SO4 |
| Molar mass | 403.51 g/mol |
| Appearance | Brownish yellow to dark brown hygroscopic liquid |
| Odor | Tobacco-like |
| Density | 1.19 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.4 |
| Vapor pressure | 0.000098 Pa (at 25 °C) |
| Acidity (pKa) | pKa = 8.02 |
| Basicity (pKb) | 6.17 |
| Magnetic susceptibility (χ) | -48.6e-6 cm³/mol |
| Refractive index (nD) | 1.503 |
| Viscosity | Viscous Liquid |
| Dipole moment | 3.46 D |
| Thermochemistry | |
| Std enthalpy of combustion (ΔcH⦵298) | Std enthalpy of combustion (ΔcH⦵298) of Nicotine Sulphate: -13524 kJ/mol |
| Pharmacology | |
| ATC code | N07BA01 |
| Hazards | |
| Main hazards | Toxic if swallowed, inhaled, or absorbed through skin; causes skin and eye irritation; harmful to aquatic life. |
| GHS labelling | GHS02, GHS06, GHS07, GHS09 |
| Pictograms | GHS06,GHS09 |
| Signal word | Danger |
| Hazard statements | H301: Toxic if swallowed. H311: Toxic in contact with skin. H410: Very toxic to aquatic life with long lasting effects. |
| Precautionary statements | P261, P264, P270, P271, P273, P280, P301+P310, P302+P352, P304+P340, P308+P311, P330, P361, P363, P391, P405, P501 |
| NFPA 704 (fire diamond) | 3-3-0 |
| Flash point | 79°C |
| Autoignition temperature | 280°C |
| Explosive limits | Not explosive |
| Lethal dose or concentration | Lethal dose or concentration (LD50) of Nicotine Sulphate: "50 mg/kg (oral, rat) |
| LD50 (median dose) | 50-60 mg/kg |
| NIOSH | 8016 |
| PEL (Permissible) | 0.3 mg/m³ |
| REL (Recommended) | 0.03% |
| IDLH (Immediate danger) | 5 mg/m³ |
| Related compounds | |
| Related compounds |
Nicotine Nicotine polacrilex Nicotine hydrogen tartrate Nicotine bitartrate Nornicotine |